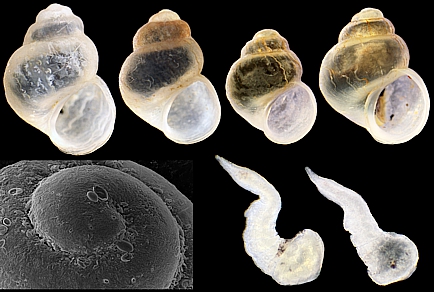
Abstract
The genus Radomaniola Szarowska, 2006 (Horatiinae) groups minute gastropods inhabiting mostly springs, but also streams, rivers and subterranean waters in the Balkans, and which are widely spread in the former Yugoslavia, but were recorded also from Italy and Greece. Thirty-six valid species distinguished so far still represent only a part of the real biodiversity within the genus. Wide morphological variability forces the application of molecular characters in the taxonomy of the genus. Cytochrome c oxidase subunit I (COI) is the most widely used marker, appropriate for genus level identification, but not sufficient for the reconstruction of deeper nodes in the phylogeny. Our materials were collected for more than 20 years: from 2001 to 2022, at 101 localities in Croatia, Bosnia and Herzegovina, Montenegro, Albania, and Greece, with 67 of them not previously studied. Many COI haplotypes have not been recorded previously. In the present paper we describe five molecularly well supported species new to science, found at 21 localities in Croatia, Bosnia and Herzegovina, and Greece, considering both morphological and molecular (COI) data. The shells, female reproductive organs and penes are described and illustrated. Wide intraspecies variability, coupled with slight differences between the taxa, were observed in the shells, penes, and female reproductive organs, suggesting morphostatic evolution in the genus Radomaniola.
References
- Bou, C. & Rouch, R. (1967) Un nouveau champ de recherches sur la faune aquatique souterraine. Comptes Rendus de l’Académie des Sciences, Series III—Sciences de la Vie, 265, 369–370.
- Cameron, R.A.D. (1992) Land snail faunas of the Napier and Oscar ranges, Western Australia: diversity, distribution and speciation. Biological Journal of the Linnean Society, 45, 271–286. https://doi.org/10.1111/j.1095-8312.1992.tb00644.x
- Cameron, R.A.D., Cook, L.M. & Hallows, J.D. (1996) Land snails on Porto-Santo: adaptive and non adaptive radiation. Philosophical Transactions of the Royal Society B, 351, 309–327. https://doi.org/10.1098/rstb.1996.0025
- Davis, G.M. (1993) Evolution of Prosobranch Snails Transmitting Asian Schistosoma; Coevolution with Schistosoma: A Review. In: Sun, T. (Ed.), Progress in Clinical Parasitology. Springer, New York, New York, pp. 145–204. https://doi.org/10.1007/978-1-4612-2732-8_6
- Delicado, D. & Hauffe, T. (2022) Shell features and anatomy of the springsnail genus Radomaniola (Caenogastropoda: Hydrobiidae) show a different pace and mode of evolution over five million years. Zoological Journal of the Linnean Society, 196, 393–441. https://doi.org/10.1093/zoolinnean/zlab121
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340
- Eschner, A. (2008) Georg von Frauenfeld: Die Bedeutung seiner Arbeiten für die Malakologie. Annales Naturhistorische Museum Wien, 109 B, 15–31.
- Falniowski, A. (1990) Anatomical characters and SEM structure of radula and shell in the species-level taxonomy of freshwater prosobranchs (Mollusca: Gastropoda: Prosobranchia): a comparative usefulness study. Folia Malacologica, 4, 53–142, 78 tabs. https://doi.org/10.12657/folmal.004.005
- Falniowski, A. (2003) Metody numeryczne w taksonomii [Numerical techniques in taxonomy]. Wydawnictwo Uniwersytetu Jagiellońskiego, Kraków, 233 pp.
- Falniowski, A. (2018) Species distinction and speciation in hydrobioid gastropods (Mollusca: Caenogastropoda: Truncatelloidea). Archives of Zoological Studies, 1, 003. https://doi.org/10.24966/AZS-7779/100003
- Falniowski, A., Georgiev, D., Osikowski, A. & Hofman, S. (2016) Radiation of Grossuana Radoman, 1973 (Caenogastropoda: Truncatelloidea) in the Balkans. Journal of Molluscan Studies, 82, 305–313. https://doi.org/10.1093/mollus/eyv062
- Falniowski, A., Lewarne, B., Rysiewska, A., Osikowski, A. & Hofman, S. (2021) Crenobiont, stygophile and stygobiont molluscs in the hydrographic area of the Trebišnjica River Basin. ZooKeys, 1047, 61–89. https://doi.org/10.3897/zookeys.1047.64034
- Falniowski, A. & Szarowska, M. (2011) Radiation and phylogeography in a spring snail Bythinella (Mollusca: Gastropoda: Rissooidea) in continental Greece. Annales Zoologici Fennici, 48, 67–90. [https://www.jstor.org/stable/23737066] https://doi.org/10.5735/086.048.0201
- Falniowski, A., Szarowska, M., Glöer, P. & Pešić, V. (2012) Molecules vs morphology in the taxonomy of the Radomaniola/Grossuana group of Balkan Rissooidea (Mollusca: Caenogastropoda). Journal of Conchology, 41, 19–36.
- Frauenfeld, G. von. (1863) Vorläufige Aufzählung der Arten der Gattungen Hydrobia Htm. und Amnicola Gld. Hldm. in der kaiserlichen und in Cuming’s Sammlung. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien, 13, 1017–1032. [https://www.biodiversitylibrary.org/page/54758154]
- Gittenberger, E. (1991) What about non-adaptive radiation? Biological Journal of the Linnean Society, 43, 263–272. https://doi.org/10.1111/j.1095-8312.1991.tb00598.x
- Glöer, P. (2022) The freshwater gastropods of the West Palaearctis. Identification key, Anatomy, Ecology, Distribution. III. Hydrobiidae. The author, Hetlingen, 528 pp.
- Gray, J.E. (1840) A manual of the land and freshwater shells of the British Islands by W. Turton. Longman, Rees, Orme, Brown and Green, London, 324 pp.
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Hershler, R. & Ponder, W.F. (1998) A review of morphological characters of hydrobioid snails. Smithsonian Contributions to Zoology, 600, 1–55. https://doi.org/10.5479/si.00810282.600
- Hofman, S., Grego, J., Beran, L., Jaszczyńska, A., Osikowski, A. & Falniowski, A. (2022) Kerkia Radoman, 1978 (Caenogastropoda: Hydrobiidae): endemism, apparently morphostatic evolution and cryptic speciation. Molluscan Research, 42, 295–319. https://doi.org/10.1080/13235818.2022.2129943
- Hütter, T., Ganser, M.H., Kocher, M., Halkic, M., Agatha, S. & Augsten, N. (2020) DeSignate: detecting signature characters in gene sequence alignments for taxon diagnoses. BMC Bioinformatics, 21, 151. https://doi.org/10.1186/s12859-020-3498-6
- Kozlov, A.M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. (2019) RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics, 35 (21), 4453–4455. https://doi.org/10.1093/bioinformatics/btz305
- Kumar, S., Stecher, G. & Tamura, K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054
- Küster, H.C. (1852-1853) Die Gattungen Paludina, Hydrocaena und Valvata. In: Abbildungen nach der Natur mit Beschreibungen. Systematisches Conchylien-Cabinet von Martini und Chemnitz. Bd. 1. Abt. 21. 1 (21) (113), (115) & (119). 2nd Edition. Bauer & Raspe, Nürnberg, pp. 1–24, pls. 1 + 2 (1852), pp. 25–56, pls. 3–8 (1852), pp. 57–96, pls. 9–14 (1853). [https://biodiversitylibrary.org/page/34226359]
- Metsalu, T. & Vilo, J. (2015) ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Research, 43, 566–570. https://doi.org/10.1093/nar/gkv468
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, 2010, 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Nylander, J.A.A. (2004) MrModeltest. Version 2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala. [program]
- Peres-Neto, P.R., Jackson, D.A. & Somers, K.M. (2005) How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Computational Statistics and Data Analysis, 49, 974–997. https://doi.org/10.1016/j.csda.2004.06.015
- Prié, V. & Bichain, J-M. (2009) Phylogenetic relationships and description of a new stygobite species of Bythinella (Mollusca, Gastropoda, Caenogastropoda, Amnicolidae) from southern France. Zoosystema, 31, 987–1000. https://doi.org/10.5252/z2009n4a12
- Prié, V. & Cucherat, X. (2021) Additional molecular data on the protected springsnail species Bythinella viridis (Poiret, 1801) (Gastropoda: Bythinellidae) suggest synonymy of related taxa. Knowledge and Management of Aquatic Ecosystems, 422, 36. https://doi.org/10.1051/kmae/2021035
- Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. (2011) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology, 21, 1864–1877. https://doi.org/10.1111/j.1365-294x.2011.05239.x
- Puillandre, N., Brouillet, S. & Achaz, G. (2021) ASAP: assemble species by automatic partitioning. Molecular Ecology Resources, 21, 609–620. https://doi.org/10.1111/1755-0998.13281
- Radoman, P. (1973) New classification of fresh and brackish water Prosobranchia from the Balkans and Asia Minor. Posebna Izdanja Prirodnogo muzeja u Beogradu, 32, 1–30.
- Radoman, P. (1978) Neue Vertreter der Gruppe Hydrobioidea von der Balkanhalbinsel. Archiv für Molluskenkunde, 109, 27–43, pls. 4–5.
- Radoman, P. (1983) Hydrobioidea a superfamily of Prosobranchia (Gastropoda). I. Systematics. Monographs Serbian Academy of Sciences and Arts, DXLVII, Department Sciences, 57, 1–256.
- Radoman, P. (1985) Hydrobioidea, a superfamily of Prosobranchia (Gastropoda). II. Origin, zoogeography, evolution in the Balkans and Asia Minor. Faculty of Science – Department of Biology Monographs, 1, Institute of Zoology Beograd, 1, 1–173.
- Rambaut, A. (2010) FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh.
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901. https://doi.org/10.1093/sysbio/syy032
- Rohlf, F.J. (1998) NTSYSpc. Numerical taxonomy and multivariate analysis system. Version 2.0. Exeter Software, Setauket, New York. [computer software and manual].
- Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Hohn, A.S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
- Rueden, D.T., Schindelin, J., Hiner, M.C., Dezonia, B.E., Walter, A.E., Arena, E.T. & Eliceiri, K.W. (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics, 18, e529. https://doi.org/10.1186/s12859-017-1934-z
- Schütt, H. (1961) Weitere neue Süßwasser-Höhlenschnecken aus Dalmatien. Archiv für Molluskenkunde, 90, 139–144.
- Stimpson, W. (1865) Diagnoses of newly discovered genera of gasteropods, belonging to the sub-fam. Hydrobiinae of the family Rissoidae. American Journal of Conchology, 1 (1), 52–54. [https://www.biodiversitylibrary.org/page/1608457]
- Szarowska, M. (1996) The egg capsules of Bythinella austriaca (Frauenfeld, 1856) with observations on the veliger and embryonic shell. Journal of Molluscan Studies, 62, 546–549. https://doi.org/10.1093/mollus/62.4.546
- Szarowska, M. (2006) Molecular phylogeny, systematics and morphological character evolution in the Balkan Rissooidea (Caenogastropoda). Folia Malacologica, 14 (3), 99–168. https://doi.org/10.12657/folmal.014.014
- Szarowska, M. & Falniowski, A. (2008) There is no philosopher’s stone: coup de grace for the morphology-based systematics in the rissooidean gastropods? 5th Congress of the European Malacological Societies, Ponta Delgada, 2008, 28.
- Szarowska, M., Osikowski, A., Hofman, S. & Falniowski, A. (2016) Pseudamnicola Paulucci, 1878 (Caenogastropoda: Truncatelloidea) from the Aegean Islands: a long or short story? Organisms Diversity and Evolution, 16, 121–139. https://doi.org/10.1007/s13127-015-0235-5
- Taylor, D.W. (1966) A remarkable snail fauna from Coahuila, México. Veliger, 9 (2), 152–228.
- Wagner, A.J. (1928) Studien zur Molluskenfauna der Balkanhalbinsel mit besonderer Berücksichtigung Bulgariens und Thraziens, nebst monographischer Bearbeitung einzelner Gruppen. Prace Zoologiczne Polskiego Państwowego Muzeum Przyrodniczego [Annales Zoologici Musei Polonici Historiae Naturalis], 6 (4), 263–399, pls. 10–23.
- Westerlund, C.A. (1881) Malakologiska bidrag. I. För Skandinaviens fauna nya land- och sötvatten-mollusker. II. För vetenskapen nya land- och sötvatten-mollusker. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar, Stockholm, 38 (4), 35–69.
- Westerlund, C.A. (1886) Fauna der in der paläarctischen Region (...) lebenden Binnenconchylien. Håkan Ohlsson, Lund, 156 + 13 (Register) pp. [https://biodiversitylibrary.org/page/10887359] https://doi.org/10.5962/bhl.title.10301
- Wilke, T. & Davis, G.M. (2000) Infraspecific mitochondrial sequence diversity in Hydrobia ulvae and Hydrobia ventrosa (Hydrobiidae: Rissoacea: Gastropoda): do their different life histories affect biogeographic patterns and gene flow? Biological Journal of the Linnean Society, 70, 89–105. https://doi.org/10.1111/j.1095-8312.2000.tb00202.x
- Wilke, T. & Falniowski, A. (2001) The genus Adriohydrobia (Hydrobiidae: Gastropoda): polytypic species or polymorphic populations? Journal of Zoological Systematics and Evolutionary Research, 39, 227–234. https://doi.org/10.1046/j.1439-0469.2001.00171.x
- WoRMS (2025) World Register of Marine Species. Available from: https://www.marinespecies.org (accessed 28 July 2025) https://doi.org/10.14284/170
- Xia, X. (2000) Data analysis in molecular biology and evolution. Kluwer Academic Publishers, Boston, Dordrecht and London, 296 pp.
- Xia, X. (2013) DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Molecular Biology and Evolution, 30, 1720–1728. https://doi.org/10.1093/molbev/mst064
- Xia, X., Xie, Z., Salemi, M., Chen, L. & Wang, Y. (2003) An index of substitution saturation and its application. Molecular Phylogenetics and Evolution, 26 (1), 1–7. https://doi.org/10.1016/S1055-7903(02)00326-3
- Zhang, J., Kapli, P., Pavlidis, P. & Stamatakis, A. (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics, 29, 2869–2876. https://doi.org/10.1093/bioinformatics/btt499