Abstract
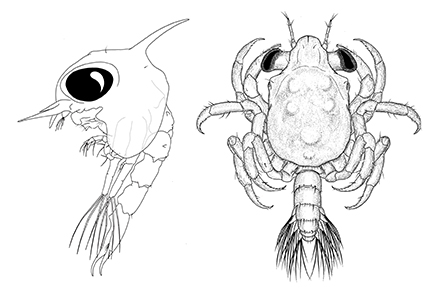
This study provides molecular and morphological evidence supporting the existence of two distinct Taliepus species along the Southeastern Pacific coast, T. dentatus (H. Milne Edwards, 1834) and T. marginatus (Bell, 1836). DNA analysis revealed a significant genetic divergence between the two species, with a 95% similarity in a partial 16S rRNA sequence. The observed genetic distance falls within the expected intrageneric range, confirming the validity of both species. In addition, the complete larval development of T. marginatus is described and illustrated for the first time and compared with that for T. dentatus (by Fagetti & Campodonico 1971). While some morphological differences were observed between the two species, these were minor and may not be sufficient for reliable identification. Identification keys for zoeal and megalopa stages of the subfamily Epialtinae Macleay, 1838 were provided, aiding in the identification of plankton-collected specimens. The limited morphological divergence between the two Taliepus species suggests that speciation may have been driven by ecological and/or environmental factors affecting the adult stage.
References
- Aedo, G., Retamal, M.A., Suárez, C., Montecinos, S., Gacitúa, S., Pedraza, M. & Arana, P. (2009) Estado actual del conocimiento de las principales especies de jaibas. Proyecto del Fondo de investigación Pesquera No 2007-39. IFOP, Concepción, 248 pp.
- Alcock, A. (1895) Materials for a carcinological fauna of India. No. 1. The Brachyura Oxyrhyncha. Journal of the Asiatic Society of Bengal, 64 (2), 157–291, pls. III–V. https://doi.org/10.5962/bhl.title.16033
- Almanza, V., Buschmann, A.H., Hernández-González, M.C. & Henríquez, L.A. (2012) Can giant kelp (Macrocystis pyrifera) forests enhance invertebrate recruitment in southern Chile? Marine Biology Research, 8 (9), 855–864. https://doi.org/10.1080/17451000.2012.692159
- Almeida-Silva, D. & Vera Candioti, F. (2024) Shape Evolution in Two Acts: Morphological Diversity of Larval and Adult Neoaustraranan Frogs. Animals, 14 (10), 1406. https://doi.org/10.3390/ani14101406
- Antezana, T., Fagetti, E. & López, M.T. (1965) Observaciones bioecológicas en decápodos comunes de Valparaíso. Revista de Biología Marina, Valparaíso, 12 (1), 2.
- Baldanzi, S., Saldías, G.S., Vargas, C.A. & Porri, F. (2022) Long term environmental variability modulates the epigenetics of maternal traits of kelp crabs in the coast of Chile. Scientific Reports, 12 (1), 18806. https://doi.org/10.1038/s41598-022-23165-1
- Baldanzi, S., Storch, D., Fusi, M., Weidberg, N., Tissot, A., Navarrete, S. & Fernández, M. (2020) Combined effects of temperature and hypoxia shape female brooding behaviors and the early ontogeny of the Chilean kelp crab Taliepus dentatus. Marine Ecology Progress Series, 646, 93–107. https://doi.org/10.3354/meps13381
- Baldanzi, S., Storch, D., Navarrete, S.A., Graeve, M. & Fernández, M. (2018) Latitudinal variation in maternal investment traits of the kelp crab Taliepus dentatus along the coast of Chile. Marine Biology, 165 (2), 37. https://doi.org/10.1007/s00227-018-3294-2
- Bell, T. (1836) Some account of the Crustacea of the coasts of South America, with descriptions of new genera and species; founded principally on the collections obtained by Mr. Cuming and Mr. Miller. (Tribus 1, Oxyrhynchi.). Proceedings of the Zoological Society of London, 1835 (35), 169–173.
- Carreja, B., Fernández, M. & Agustí, S. (2016) Joint additive effects of temperature and UVB radiation on zoeae of the crab Taliepus dentatus. Marine Ecology Progress Series, 550, 135–145. https://doi.org/10.3354/meps11715
- Christy, J.H. (1987) Competitive mating, mate choice and mating associations of brachyuran crabs. Bulletin of Marine Science, 41 (2), 177–191.
- Clark, P.F. (1983) The larval and first crab stages of the three Inachus species (Crustacea, Decapoda, Majidae); a morphological and statistical analysis. Bulletin of the British Museum (Natural History), Zoology, 44 (2), 179–190.
- Clark, P.F. (1984) A comparative study of zoeal morphology in the genus Liocarcinus (Crustacea, Brachyura, Portunidae). Zoological Journal of the Linnean Society, 82, 273–290. https://doi.org/10.1111/j.1096-3642.1984.tb00644.x
- Clark, P.F., Calazans, D.K. & Pohle, G.W. (1998) Accuracy and standardization of brachyuran larval descriptions. Invertebrate Reproduction and Development, 33 (2–3), 127–144. https://doi.org/10.1080/07924259.1998.9652627
- Clark, P.F. & Cuesta, J.A. (2015) Larval systematics of Brachyura. In: Castro, P., Davie, P., Guinot, D., Schram, F. & Klein, C.V. (Eds.), Treatise on Zoology - Anatomy, Taxonomy, Biology - The Crustacea complementary to the Traité de Zoologie. Vol. 9C-II. Brill, New York, New York, pp. 981–1048. https://doi.org/10.1163/9789004190832_020
- Colavite, J., Santana, W. & Pohle, G. (2014) Larval development of the spider crab Menaethius monoceros (Latreille, 1825), (Crustacea: Decapoda: Brachyura: Epialtidae). Journal of Natural History, 48 (37–38), 2273–2292. https://doi.org/10.1080/00222933.2014.925596
- Crandall, K.A. & Fitzpatrick, J.F. (1996) Crayfish Molecular Systematics: Using a Combination of Procedures to Estimate Phylogeny. Systematic Biology, 45 (1), 1–26. https://doi.org/10.1093/sysbio/45.1.1
- Dana, J.D. (1851) Conspectus crustaceorum quae in orbis terrarum circumnavigatione, Carolo Wilkes e classe Reipublicae Faederatae duce, lexit et descripsit. Pars VI. [A survey of the crustaceans which took place in the circumnavigation of the world, under the command of Charles Wilkes of the United States Navy, he read and described. Part 6]. The American Journal of Science and Arts, Series 2, 11 (32), 268–274.
- DecaNet Eds. (2024) DecaNet. World Register of Marine Species. Available from: https://www.marinespecies.org (accessed 24 October 2024)
- De Haan, W. (1837) Crustacea. In: von Siebold, P.F. (Ed.), Fauna Japonica sive Descriptio Animalium, quae in Itinere per Japoniam, Jussu et Auspiciis Superiorum, qui Summum in India Batava Imperium Tenent, Suspecto, Annis 1823-1830 Collegit, Notis, Observationibus et Adumbrationibus Illustravit. Apud Auctorem, Lugduni Batavorum, pp. i–xxxi + ix–xvi + 1–243, pls. A–J + L–Q + 1–55.
- Eirin-Lopez, J.M. & Putnam, H.M. (2019) Marine Environmental Epigenetics. Annual Review of Marine Science, 11 (1), 335–368. https://doi.org/10.1146/annurev-marine-010318-095114
- Estoup, A., Largiader, C., Perrot, E. & Chourrout, D. (1996) Rapid one-tube DNA extraction for reliable PCR detection of fish polymorphic markers and transgenes. Molecular Marine Biology and Biotechnology, 5, 295–298.
- Fagetti, E. & Campodonico, I. (1971) Desarrollo larval en el laboratorio de Taliepus dentatus (Milne-Edwards) (Crustacea Brachyura: Majidae, Acanthonychinae). Revista de Biología Marina, Valparaiso, 14 (3), 1–14.
- Fariña, J.M. & Ojeda, F.P. (1993) Abundance, activity, and trophic patterns of the redspotted catshark, Schroederichthys chilensis, on the Pacific temperate coast of Chile. Copeia, 2, 545–549. https://doi.org/10.2307/1447159
- Fellous, A., Andrade, S., Vidal-Ramirez, F., Calderón, R., Beltran, J. & Correa, J.A. (2017) Modulatory effect of the exudates released by the brown kelp Lessonia spicata on the toxicity of copper in early developmental stages of ecologically related organisms. Environmental Science and Pollution Research, 24 (4), 3900–3911. https://doi.org/10.1007/s11356-016-8120-0
- Fernández, M., Brante, A. & Baldanzi, S. (2020) Costs and benefits of brooding among decapod crustaceans: The challenges of incubating in aquatic systems. In: The Natural History of the Crustacea: Reproductive Biology. Vol. VI. Oxford University Press, New York, New York, pp. 86–114. https://doi.org/10.1093/oso/9780190688554.003.0004
- Garth, J.S. & Abbott, D.P. (1980) Brachyura: The True Crabs. In: Morris, R.H., Abbott, D.P. & Haderlie, E.C. (Eds.), Intertidal Invertebrates of California. Stanford University Press, Stanford, pp. 594–630.
- Ginsburg, I. (1933) Descriptions of five new species of seahorses. Journal of the Washington Academy of Sciences, 23 (12), 560–563.
- Greville, R.K. (1830) Algae britannicae, or descriptions of the marine and other inarticulated plants of the British islands, belonging to the order Algae; with plates illustrative of the genera. Maclachlan & Stewart, Edinburgh, 218 pp.
- Guichenot, A. (1848) Fauna Chilena. Peces. In: Gay, C. (Ed.), Historia, física y política de Chile. Zoología. Vol. 2. En casa del autor, Santiago, pp. 137–370.
- Guinot, D. & Richer de Forges, B. (1985) Revision of the Indo-Pacific Sphenocarcinus with a single rostrum and description of two new species (Crustacea, Decapoda, Brachyura, Majidae). Marine Research in Indonesia, 2, 49–71, pls. I–II. https://doi.org/10.14203/mri.v24i0.400
- Hofmann, G.E. (2017) Ecological Epigenetics in Marine Metazoans. Frontiers in Marine Science, 4, 4. https://doi.org/10.3389/fmars.2017.00004
- Hultgren, K.M. & Stachowicz, J.J. (2008) Molecular phylogeny of the brachyuran crab superfamily Majoidea indicates close congruence with trees based on larval morphology. Molecular Phylogenetics and Evolution, 48 (3), 986–996. https://doi.org/10.1016/j.ympev.2008.05.004
- Hultgren, K.M. & Stachowicz, J.J. (2010) Size-related habitat shifts facilitated by positive preference induction in a marine kelp crab. Behavioral Ecology, 21 (2), 329–336. https://doi.org/10.1093/beheco/arp192
- Jablonka, E. & Lamb, M.J. (1998) Epigenetic inheritance in evolution. Journal of Evolutionary Biology, 11 (2), 159–183. https://doi.org/10.1046/j.1420-9101.1998.11020159.x
- Jofré Madariaga, D., Ortiz, M. & Thiel, M. (2013) Demography and feeding behavior of the kelp crab Taliepus marginatus in subtidal habitats dominated by the kelps Macrocystis pyrifera or Lessonia trabeculata. Invertebrate Biology, 132 (2), 133–144. https://doi.org/10.1111/ivb.12021
- Ko, H.S. (1998) Zoeal development of three species of Pugettia (Decapoda: Majidae), with a key to the known zoeas of the subfamily epialtinae. Journal of Crustacean Biology, 18 (3), 499–510. https://doi.org/10.2307/1549414
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution, 35 (6), 1547–1549. https://doi.org/10.1093/molbev/msy096
- Kützing, F.T. (1843) Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. F.A. Brockhaus, Leipzig, xxxii + 459 pp., 80 pls. [Part 1: pp (i)–xxxii + (1)–142, Part 2: pp. 143–458 + 1, err, pls. 1–80] https://doi.org/10.5962/bhl.title.4746
- Latreille, P.A. (1825) Histoire naturelle. Entomologie, ou histoire naturelle des Crustaces, des Arachnides et des Insects. Agasse Imprimeur-Libraire, Paris, 833 pp. [pp. 1–344 (published 1 October 1825) + 345–832 + errata (published 13 December 1828)]
- Lamarck, J.-B.M. de (1819) Histoire naturelle des animaux sans vertèbres. Tome sixième, 1re partie, Paris, vi + 343 pp.
- Leach, W.E. (1820) Malacostraca Podophthalmata Britanniae; or descriptions of such British species of the Linnean genus Cancer as have their eyes elevated on footstalks. Illustrated with figures of all species by John Sowerby, F.L.S. G.S. & W.S. &c. John Sowerby, Lambeth, London, 124 pp., 45 pls. [Part 17 (pls. 9B + 10)].
- Lesson, R.P. (1837) Histoire naturelle des oiseaux. Vol. 9. P.H.E. Levrault, Paris. [unknown pagination]
- Lichtenstein, M.H.C. (1823) Verzeichniss der Doubletten des zoologischen Museums der Königl. Universität zu Berlin nebst Beschreibung vieler bisher unbekannter Arten von Säugethieren, Vögeln, Amphibien und Fischen, 118 pp. https://doi.org/10.5962/bhl.title.40281
- Linnaeus, C. (1771) Mantissa plantarum altera. Laurentius Salvius, Holmiae [Stockholm], 588 pp.
- MacLeay, W.S. (1838) Illustrations of the Annulosa of South Africa. On the brachyurous decapod Crustacea. Brought from the Cape by Dr. Smith. In: Smith, A. (Ed.), Illustrations of the Zoology of South Africa; consisting chiefly of Figures and Descriptions of the Objects of Natural History Collected during an Expedition into the Interior of South Africa, in the Years 1834, 1835, and 1836; fitted out by “The Cape of Good Hope Association for Exploring Central Africa”. Published under the Authority of the Lords Commissioners of Her Majesty’s Treasury, London, pp. i–iv + 53–71, pls. 2 + 3.
- Manríquez, P.H. & Cancino, J.M. (1991) Depredación de Membranipora isabellaeana (Bryozoa) por Taliepus dentatus (Crustacea: Decapada). Revista de Biología Marina, Valparaíso, 26, 309–323.
- Marshall, D.J. & Morgan, S.G. (2011) Ecological and Evolutionary Consequences of Linked Life-History Stages in the Sea. Current Biology, 21 (18), R718–R725. https://doi.org/10.1016/j.cub.2011.08.022
- Medina, I., Vega-Trejo, R., Wallenius, T., Symonds, M.R.E. & Stuart-Fox, D. (2020) From cryptic to colorful: Evolutionary decoupling of larval and adult color in butterflies. Evolution Letters, 4 (1), 34–43. https://doi.org/10.1002/evl3.149
- Medina‐Vogel, G., Delgado, C., Álvarez, P.R.E. & Bartheld, J.L. (2004) Feeding ecology of the marine otter (Lutra felina) in a rocky seashore of the south of Chile. Marine Mammal Science, 20 (1), 134–144. https://doi.org/10.1111/j.1748-7692.2004.tb01144.x
- Meyen, F.J.F. (1834) Beiträge zur Zoologie, gesammelt auf einer Reise um die Erde, auf S. M. Schiffe Prinzess Louise, commandirt von Captain W. Wendt, in den Jahren 1830, 1831 und 1832. Verhandlungen der Kaiserlichen Leopoldinisch-Carolinischen Akademie der Naturforscher, 16 (1), 551–612. https://doi.org/10.5962/bhl.title.138287
- Milne-Edwards, A. (1878) Études sur les xiphosures et les crustacés de la région mexicaine. In Mission scientifique au Mexique et dans l’Amérique centrale, ouvrage publié par odre du Ministre de l’Instruction publique. Recherches Zoologiques pour servir à l’histoire de la faune de l’Amérique centrale et du Mexique, publiées sous la direction de M. H. Milne Edwards, membre de l’Institut. Cinquième partie. Tome Premier. Imprimerie nationale, Paris, pp. 1–8 (unpaginated) + 1–368 + 1–63 (unpaginated plate legends) pp., pls. 1–61 + 5A + 31A (63 pls).
- Milne Edwards, H. (1833) Du genre Leucippe, établi d’après un crustacé nouveau de la classe des décapodes. Annales de la Société Entomologique de France, 2, 512–517, pl. 18b.
- Milne Edwards, H. (1834) Histoire Naturelle des Crustacés, Comprenant l´Anatomie, la Physiologie et la Classification de ces Animaux. Vol. I. Encyclopédique Roret, Paris, xxxv + 468 pp. https://doi.org/10.5962/bhl.title.16170
- Molina, G.I. (1782) n.k. In: Libro IV: Vermi, Insetti, Rettili, Pesci, Uccelli, e Quadrupedi del Chili. Saggio Sulla Storia Naturale del Chili. Nella Stamperia de S. Tommaso d’ Aquino, Bologna, pp. 197–367. https://doi.org/10.5962/bhl.title.62689
- Müller, J.P. & Troschel, F.H. (1840) Ueber die Classen, Ordnungen und Familien der Asterien, Ophiuren und Crinoiden. Monatsberichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin, 1840, 108–137.
- Nenen, J.P.C. (2010) Respuestas fisiológicas a la carnivoría y herbivoría durante el desarrollo bentónico del cangrejo Taliepus dentatus (Milne Edwards, 1834) (Decapoda, Majidae): una aproximación bioenergética. Universidad Austral de Chile, Valdivia, 61 pp. [pp. i–vii + 8–61]
- Nobili, G. (1905) Quatre décapodes nouveaux du Golfe Persique (récoltes de MM. J. Bonnier et Ch. Pérez). Bulletin du Muséum d’Histoire naturelle, 11, 238–239.
- Palma, A.T., Soto-Gamboa, M. & Ojeda, F.P. (2011) Ontogenetic habitat shift of an herbivorous crab: a chemically mediated defense mechanism? Revista de Biología Marina y Oceanografía, 46 (3), 329–338. https://doi.org/10.4067/S0718-19572011000300004
- Pardo, L.M., Palma, A.T., Prieto, C., Sepulveda, P., Valdivia, I. & Ojeda, F.P. (2007) Processes regulating early post-settlement habitat use in a subtidal assemblage of brachyuran decapods. Journal of Experimental Marine Biology and Ecology, 344 (1), 10–22. https://doi.org/10.1016/j.jembe.2006.12.024
- Pretterebner, K. & Pardo, L.M. (2020) All or nothing: Switch to high current reproductive investment under risk of starvation in male kelp crab. Ecology and Evolution, 10 (7), 3383–3391. https://doi.org/10.1002/ece3.6131
- Randall, J.W. (1840) Catalogue of the Crustacea brought by Thomas Nutall and J.K. Townsend, from the West Coast of North America and the Sandwich Islands, with descriptions of such species as are apparently new, among which are included several species of different localities, previously existing in the collection of the Academy. Journal of the Academy of Natural Sciences at Philadelphia, 8, 106–147, pls. 3–7. https://doi.org/10.5962/bhl.title.119921
- Retamal, M.A. (1995) Decápodos de Chile. World Biodiversity Database CD-ROM Series. ETI-U. de Concepción. Springer-Verlag. Available from: https://decapodos-chile.linnaeus.naturalis.nl/linnaeus_ng/app/views/introduction/topic.php?id=3347&epi=119 (accessed 30 December 2020)
- Retamal, M.A., Aedo, G., Suárez, C., Montecinos, S., Gacitúa, S., Pedraza, M. & Arana, P. (2009) Estado actual del conocimiento de las principales especies de jaibas a nivel nacional. Fondo de Investigación Pesquera, Informe Técnico FIP-IT/2007-39, 1–237.
- Retamal, M.A. & Moyano, H.I. (2010) Zoogeografía de los crustáceos decápodos chilenos marinos y dulceacuícolas. Latin American Journal of Aquatic Research, 38 (3), 302–328. https://doi.org/10.3856/vol38-issue3-fulltext-1
- Rice, A.L. (1980) Crab zoeal morphology and its bearing on the classification of the Brachyura. Transactions of the Zoological Society of London, 35, 271–424. https://doi.org/10.1111/j.1096-3642.1980.tb00060.x
- Rice, A.L. (1988) The megalopa stage in majid crabs, with a review of spider crab relationships based on larval characters. In: Fincham, A.A. & Rainbow, P.S. (Eds.), Aspects of Decapod Crustaceans Biology. The Proceedings of a Symposium held at the Zoological Society of London on 8 and 9 April 1987. Symposia of the Zoological Society of London. Oxford Science Publications Issue 59. Clarendon Press, Oxford, pp. 27–46.
- Samouelle, G. (1819) The entomologists’ useful compendium; or an introduction to the knowledge of British Insects, comprising the best means of obtaining and preserving them, and a description of the apparatus generally used; together with the genera of Linné, and modern methods of arranging the Classes Crustacea, Myriapoda, spiders, mites and insects, from their affinities and structure, according to the views of Dr. Leach. Also an explanation of the terms used in entomology; a calendar of the times of appearance and usual situations of near 3,000 species of British Insects; with instructions for collecting and fitting up objects for the microscope. Thomas Boys, London, 496 pp., 12 pls. https://doi.org/10.5962/bhl.title.120094
- Santelices, B. (2012) Lessonia spicata. In: González, A., Beltrán, J., Hiriart-Bertrand, L., Flores, V., de Reviers, B., Correa, J.A. & Santelices, B., Identification of cryptic species in the Lessonia nigrescens complex (Phaeophyceae, Laminariales). Journal of Phycology, 48, 1153–1165. https://doi.org/10.1111/j.1529-8817.2012.01200.x
- Schubart, C.D., Cuesta, J.A. & Felder, D.L. (2002) Glyptograpsidae, a new brachyuran family from Central America: larval and adult morphology, and a molecular phylogeny of the Grapsoidea. Journal of Crustacean Biology, 22, 28–44. https://doi.org/10.1163/20021975-99990206
- Stimpson, W. (1857) On the Crustacea and Echinodermata of the Pacific shores of North America. Boston Journal of Natural History, 6, 444–532, pls. 18–23. [https://archive.org/stream/crustaceaechinod00stim#page/n3/mode/2up] https://doi.org/10.5962/bhl.title.59693
- Storch, D., Fernández, M., Navarrete, S. & Pörtner, H. (2011) Thermal tolerance of larval stages of the Chilean kelp crab Taliepus dentatus. Marine Ecology Progress Series, 429, 157–167. https://doi.org/10.3354/meps09059
- Storch, D., Santelices, P., Barria, J., Cabeza, K., Pörtner, H.-O. & Fernández, M. (2009) Thermal tolerance of crustacean larvae (zoea I) in two different populations of the kelp crab Taliepus dentatus (Milne-Edwards). Journal of Experimental Biology, 212 (9), 1371–1376. https://doi.org/10.1242/jeb.030205
- Strader, M.E., Kozal, L.C., Leach, T.S., Wong, J.M., Chamorro, J.D., Housh, M.J. & Hofmann, G.E. (2020) Examining the Role of DNA Methylation in Transcriptomic Plasticity of Early Stage Sea Urchins: Developmental and Maternal Effects in a Kelp Forest Herbivore. Frontiers in Marine Science, 7. [published online] https://doi.org/10.3389/fmars.2020.00205
- Suarez-Bregua, P., Rosendo, S., Comesaña, P., Sánchez-Ruiloba, L., Morán, P., Planas, M. & Rotllant, J. (2021) Dynamic changes in DNA methylation during seahorse (Hippocampus reidi) postnatal development and settlement. Frontiers in Zoology, 18 (1), 52. https://doi.org/10.1186/s12983-021-00436-7
- Valenzuela-Carvajal, P., Rojas-Hernandez, N., Veliz, D., Fibla, P. & Pardo, L.M. (2024) The complete mitochondrial genome and microsatellite discovery of the Chilean kelp crab Taliepus dentatus (Brachuyra, Majoidea). Crustaceana, 97 (5–9), 535–551. https://doi.org/10.1163/15685403-bja10400
- Vásquez, J.A. & Alonso Vega, J.M. (2001) Chondracanthus chamissoi (Rhodophyta, Gigartinales) in northern Chile: ecological aspects for management of wild populations. Journal of Applied Phycology, 13 (3), 267–277. https://doi.org/10.1023/A:1011152922832
- Villouta, E. & Santelices, B. (1986) Lessonia trabeculata sp. nov. (Laminariales, Phaeophyta), a new kelp from Chile. Phycologia, 25, 81–86. https://doi.org/10.2216/i0031-8884-25-1-81.1
- Weber, F. (1795) Nomenclator entomologicus secundum Entomologiam systematicum ill. Fabricii adjectis speciebus recens detectis et varietatibus. Apud Carolum Ernestum Bohn, Chilonii et Hamburgii, 93 pp. https://doi.org/10.5962/bhl.title.12297
- Zagal, C. & Hermosilla, C. (2007) Guía de los invertebrados marinos del Sur de Chile. 2nd Edition. Editorial Fantástico Sur, Punta Arenas, 263 pp.