Abstract
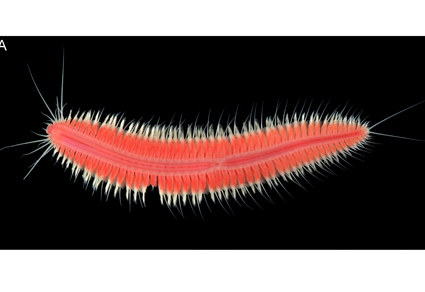
A new, brightly red-coloured, symbiotic hesionid worm, Parahesione dudahamra sp. nov., is described based on the holotype and single specimen collected in the shore waters of the King Abdullah University of Science and Technology, Thuwal, Red Sea coast of Saudi Arabia. The new species is characterized by simple lateral antennae without distinct ceratophores, longest dorsal cirri reaching chaetiger 12, and longest ventral cirri reaching only chaetiger 4. The holotype was extracted from a burrow of an unknown host in very shallow water, close to mangrove roots. The diversified burrowing fauna of the type locality, including the possible infaunal hosts of P. dudahamra sp. nov., is briefly discussed. In addition, a full description and ecological notes are provided for another hesionid worm, Leocrates giardi Gravier, 1900, of which several specimens were extracted from burrows at the type locality of P. dudahamra sp. nov.
References
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990) Basic local alignment search tool. Journal of Molecular Biology, 215 (3), 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
- Anderson, S.J., Atkinson, R.J.A. & Taylor, A.C. (1991) Behavioural and respiratory adaptations of the mud-burrowing shrimp Calocaris macandreae Bell (Thalassinidea: Crustacea) to the burrow environment. Ophelia, 34 (2), 143–156. https://doi.org/10.1080/00785326.1991.10429702
- Anker, A., Murina, G.V., Lira, C., Vera Caripe, J.A., Palmer, A.R. & Jeng, M.S. (2005) Macrofauna associated with echiuran burrows: a review with new observations of the innkeeper worm, Ochetostoma erythrogrammon Leuckart and Rüppel, in Venezuela. Zoological Studies, 44 (2), 157–190.
- Atkinson, R.J.A. & Taylor, A.C. (2005) Aspects of the physiology, biology and ecology of thalassinidean shrimps in relation to their burrow environment. In: Gibson, R.N., Atkinson, R.J.A. & Gordon, J.D.M. (Eds.), Oceanography and Marine Biology: An Annual Review. CRC Press, Boca Raton, Florida, pp. 183–220. https://doi.org/10.1201/9781420037449-7
- Bely, A.E. & Wray, G.A. (2004) Molecular phylogeny of naidid worms (Annelida: Clitellata) based on cytochrome oxidase I. Molecular phylogenetics and evolution, 30 (1), 50–63. https://doi.org/10.1016/S1055-7903(03)00180-5
- Britayev, T.A. & Antokhina, T.I. (2012) Symbiotic polychaetes from Nhatrang Bay, Vietnam. In: Britayev, T.A. & Pavlov, D.S. (Eds.), Benthic fauna of the Bay of Nhatrang, Southern Vietnam. Vol. 2. KMK, Moscow, pp. 11–54.
- Carr, C.M., Hardy, S.M., Brown, T.M., Macdonald, T.A. & Hebert, P.D.N. (2011) A Tri-Oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PLoS ONE, 6 (7), e22232. https://doi.org/10.1371/journal.pone.0022232
- Claparède, É. (1868) Les annélides chétopodes du Golfe de Naples. Mémoires de la Société de Physique et d'Histoire Naturelle de Genève, 19 (2), 313–584. [https://www.biodiversitylibrary.org/page/14309905]
- De Man, J.G. (1916) Description of a new species of the genus Callianassa Leach and of a species of the genus Alpheus Fabr., both from the Indian archipelago. Zoologische Mededeelingen, Leiden, 2, 57–61.
- de Vaugelas, J. & de Saint Laurent, M. (1984) Premières données sur l’écologie de Callichirus laurae de Saint Laurent sp. nov. (Crustacea Decapoda Callianassidae): son action bioturbatrice sur les formations sédimentaires du golfe d’Aquaba (Mer Rouge). Comptes Rendus de l'Académie des Sciences, 3, 298, 147–152.
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Research, 32 (5), 1792–1797. https://doi.org/10.1093/nar/gkh340
- Fauvel, P. (1919) Annélides polychètes de Madagascar, de Djibouti et du Golfe Persique. Archives de Zoologie Expérimentale et Générale, 58, 315–473. [http://www.biodiversitylibrary.org/item/29986#page/497/mode/1up] https://doi.org/10.5962/bhl.part.8154
- Fauvel, P. (1933) Mission Robert Ph. Dollfus en Égypte: Annélides polychètes. Mémoires de l’Institut d’Égypte, 21, 31–83. https://archive.org/details/MIE_21/page/n23/mode/1up
- Fauvel, P. (1955) Annélides polychètes de la croisière de la Calypso en mer Rouge en 1952. Resultats Scientifique de les Campagnes de Calypso, 3, 101–120.
- Geller, J., Meyer, C., Parker, M. & Hawk, T. (2013) Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Molecular Ecology Resources, 13, 851–861. https://doi.org/10.1111/1755-0998.12138
- Giribet, G., Carranza, S., Baguna, J., Riutort, M. & Ribera, C. (1996) First molecular evidence for the existence of a Tardigrada+Arthropoda clade. Molecular Biology and Evolution, 13, 76–84. https://doi.org/10.1093/oxfordjournals.molbev.a025573
- Gonzalez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fernandez-Riverola, F. & Posada, D. (2010) ALTER: program-oriented format conversion of DNA and protein alignments. Nucleic Acids Research, 38 (Supplement 2), W14–W18. https://doi.org/10.1093/nar/gkq321
- Goto, R. (2017) The Echiura of Japan: Diversity, classification, phylogeny, and their associated fauna. In: Motokawa, M. & Kajihara, H. (Eds.), Species diversity of animals in Japan. Diversity and commonality in animals. Springer, Tokyo, pp. 513–542. https://doi.org/10.1007/978-4-431-56432-4_20
- Goto, R. & Kato, M. (2012) Geographic mosaic of mutually exclusive dominance of obligate commensals in symbiotic communities associated with a burrowing echiuran worm. Marine Biology, 159 (2), 319–330. https://doi.org/10.1007/s00227-011-1810-8
- Gravier, Ch. (1900) Contribution à l'étude des annélides polychètes de la Mer Rouge. Première partie. Nouvelles Archives du Muséum d’Histoire Naturelle de Paris, 4 (2), 137–282. [https://biodiversitylibrary.org/page/36872698]
- Grube, A.E. (1850) Die Familien der Anneliden. Archiv für Naturgeschichte, Berlin, 16 (1), 249–364. [https://biodiversitylibrary.org/page/6958350
- Jimi, N., Nakajima, H., Sato, T., Gonzalez, B.C., Woo, S.P., Rouse, G.W. & Britayev, T. (2023) Two new species of Parahesione (Annelida: Hesionidae) associated with ghost shrimps (Crustacea: Decapoda) and their phylogenetic relationships. PeerJ, 11, e16346. https://doi.org/10.7717/peerj.16346]
- Kinberg, J.G.H. (1866) Annulata Nova. Continuatio. [various Errantia & Sedentaria]. Öfversigt af Königlich Vetenskapsakademiens förhandlingar, Stockholm, 22 (4), 239–258. https://biodiversitylibrary.org/page/32339515
- Kozlov, A.M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. (2018) RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. BioRxiv. https://doi.org/10.1101/447110
- Lobo, J., Costa, P.M., Teixeira, M.A., Ferreira, M.S.G, Costa, M.H. & Costa, F.O. (2013) Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecology, 13 (34). [published online] https://doi.org/10.1186/1472-6785-13-34
- Marin, I. & Antokhina, T. (2020) Hidden burrow associates: macrosymbiotic assemblages of subtidal deep-burrowing invertebrates in the northern part of the Sea of Japan. Marine Biodiversity, 50 (4), 50. https://doi.org/10.1007/s12526-020-01065-9
- Martin, D. & Britayev, T. (1998) Symbiotic polychaetes: review of known species. Oceanography and Marine Biology. An Annual Review, 36, 217–340. https://doi.org/10.1201/b12646
- Martin, D. & Britayev, T.A. (2018) Symbiotic polychaetes revisited: an update of the known species and relationships (1998–2017). Oceanography and Marine Biology. An Annual Review, 56, 371–448. https://doi.org/10.1201/9780429454455-6
- Monro, C.C.A. (1926) Polychaeta of the ‘Alert’ Expedition. Families Hesionidae and Nereidae. Zoological Journal of the Linnean Society, 36 (243), 311–323. https://doi.org/10.1111/j.1096-3642.1926.tb02172.x
- Milne-Edwards, A. (1870) Révision du genre Callianassa (Leach) et description de plusieurs espèces nouvelles de ce groupe faisant partie de la collection du Muséum. Nouvelles Archives du Muséum d’Histoire Naturelle de Paris, 6, 75–102.
- Nobili, G. (1904) Diagnoses préliminaires de vingt-huit espèces nouvelles de stomatopodes et décapodes macroures de la Mer Rouge. Bulletin du Muséum d’Histoire Naturelle, 10, 228–238.
- Osborn, K.J., Rouse, G.W., Goffredi, S.K. & Robison, B.H. (2007) Description and relationships of Chaetopterus pugaporcinus, an unusual pelagic polychaete (Annelida, Chaetopteridae). Biological Bulletin, 212 (1), 40–54. https://doi.org/10.2307/25066579
- Palumbi, S.R. (1996) Nucleic Acids II: The polymerase chain reaction. In: Hillis, D.M., Moritz, C. & Mable, B.K. (Eds.), Molecular systematic. Sinauer Associates, Sunderland, Massachusetts, pp. 205–247.
- Pettibone, M.H. (1956) Some polychaete worms of the families Hesionidae, Syllidae and Nereidae from the east coast of North America, West Indies, and Gulf of Mexico. Journal of the Washington Academy of Sciences, 46 (9), 281–294. [https://www.biodiversitylibrary.org/page/39693975]
- Pettibone, M.H. (1963) Marine polychaete worms of the New England region. I. Aphroditidae through Trochochaetidae. Bulletin of the United States National Museum, 227 (1), 1–356. [https://www.biodiversitylibrary.org/page/7870746] https://doi.org/10.5479/si.03629236.227.1
- Pillay, D. & Branch, G.M. (2011) Bioengineering effects of burrowing thalassinidean shrimps on marine soft-bottom ecosystems. Oceanography and Marine Biology: An Annual Review, 49, 137–192. https://doi.org/10.1201/b11009-5
- Rambaut, A. (2018) FigTree. Tree figure drawing tool. Version 1.4.4. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 13 July 2025)
- Rambaut, A., Drummond, A.J. & Suchard, M.A. (2018) Tracer. Version 1.6. Available from: http://tree.bio.ed.ac.uk/software/tracer/ (accessed 13 July 2025)
- Ronquist, F., Huelsenbeck, J. & Teslenko, M. (2012) Draft MrBayes. Version 3.2. Manual: tutorials and model summaries. Available from: https://pubmed.ncbi.nlm.nih.gov/11524383/ (accessed 13 July 2025)
- Ruta, C., Nygren, A., Rousset, V., Sundberg, P., Tiller, A. & Wiklund, H. (2007) Phylogeny of Hesionidae (Aciculata, Polychaeta), assessed from morphology, 18S rDNA, 28S rDNA, 16S rDNA and COI. Zoologica Scripta, 36, 99–107. https://doi.org/10.1111/j.1463-6409.2006.00255.x
- Salazar-Vallejo, S.I. (2020) Revision of Leocrates Kinberg, 1866 and Leocratides Ehlers, 1908 (Annelida, Errantia, Hesionidae). Zootaxa, 4739 (1), 1–114. https://doi.org/10.11646/zootaxa.4739.1.1
- Say, T. (1818) An account of the Crustacea of the United States. Journal of the Academy of Natural Sciences of Philadelphia, 11 (2), 235–253. [https://www.biodiversitylibrary.org/page/24680585#page/303/]
- Sjölin, E., Erséus, C. & Källersjö, M. (2005) Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution, 35 (2), 431–441. https://doi.org/10.1016/j.ympev.2004.12.018
- Tamura, K., Stecher, G. & Kumar, S. (2021) MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. https://doi.org/10.1093/molbev/msab120
- Wang, Z., Qiu, J.W. & Salazar-Vallejo, S.I. (2018) Redescription of Leocrates chinensis Kinberg, 1866 (Annelida, Hesionidae). Zoological Studies, 57, e5. https://doi.org/10.6620/ZS.2018.57-05
- Webster, H.E. (1879) The Annelida Chaetopoda of New Jersey. Annual Report of the New York State Museum of Natural History, 32, 101–128. [https://www.biodiversitylibrary.org/page/35614233]
- Webster, H.E. & Benedict, J.E. (1884) The Annelida Chaetopoda from Provincetown and Wellfleet, Massachusetts. Annual Report of the United States Commission of Fish and Fisheries, Washington, 1881, 699–747. [https://www.biodiversitylibrary.org/page/11203280]
- WoRMS Editorial Board (2025) World register of marine species. Available from: https://www.marinespecies.org (accessed 25 April 2025) https://doi.org/10.14284/170