Abstract
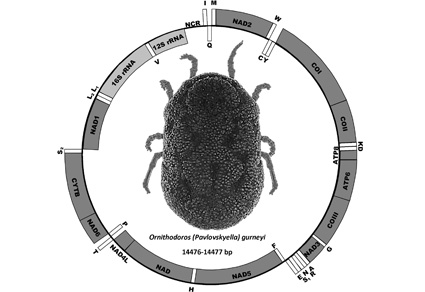
Mitochondrial (mt) genomes have played a major role in elucidating evolutionary relationships in ticks (Ixodida), especially in soft ticks (Argasidae). Mitochondrial genomes have been sequenced for representatives of most of the genera and other major lineages. This includes members of the Afrotropical, Nearctic, Neotropical and Palearctic Pavlovskyella Pospelova-Shtrom, 1950, which is a subgenus of Ornithodoros Koch, 1844. These continent-associated lineages do not form a monophyletic group: rather, the subgenus Pavlovskyella is paraphyletic. The only zoogeographic region for which mt genomes are not available is the Australasian region. Here we report the first mt genome of an Australasian Pavlovskyella: Ornithodoros (Pavlovskyella) gurneyi Warburton, 1926, the kangaroo soft tick. [The companion paper to the present work, Barker et al. (2025), presents the mt genome, 18S and 28S rRNA of the only other known Australasian Pavlovskyella, McMillan’s Australian tree-hollow argasid, Ornithodoros (Pavlovskyella) macmillani Hoogstraal & Kohls, 1966.] Our phylogenetic trees reveal that the two Australasian Pavlovskyella have their own clade (i.e. are sister-taxa) which does not have a sister-group relationship with species of Pavlovskyella from any of the four other zoogeographic regions. We propose that the Pavlovskyella from the Afrotropical, Australasian, Nearctic, Neotropical and Palearctic zoogeographic regions have independent evolutionary histories and thus are best considered different genera. Molecular dating leads us to propose that continental drift may have caused the evolution of five different lineages of Pavlovskyella, each in a different zoogeographic region of the world.
References
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990) Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
- Barker, S.C. & Barker, D. (2023) Ticks of Australasia: 125 species of ticks in and around Australia. Zootaxa, 5253 (1), 1–670. https://doi.org/10.11646/zootaxa.5253.1.1
- Barker, S.C., Kelava, S., Shaw, M.D., Teo, E.J.M., Gofton, A.W., Tan, C.J., Barker, D., Cho, M., Nakao, R., Muñoz-Leal, S. & Mans, B.J. (2025) Two new genera for the Australian species of the subgenus Pavlovskyella Pospelova-Shtrom, 1950 (genus Ornithodoros Koch, 1844) (Acari: Ixodida: Argasidae: Ornithodorinae): Apanaskevichiella n. gen. for O. (P.) macmillani (Hoogstraal & Kohls, 1966); and Australpavlovskyella n. gen. for O. (P.) gurneyi (Warburton, 1926). Zootaxa. [accepted subject to revision]
- Barros-Battesti, D.M., Onofrio, V.C., Nieri-Bastos, F.A., Soares, J.F., Marcili, A., Famadas, K.M., Faccini, J.L.H., Ramirez, D.G., Doyle, R.L., Martins, J.R., Junior, J.R., Guglielmone, A.A. & Labruna, M.B. (2012) Ornithodoros brasiliensis Aragão (Acari: Argasidae): description of the larva, redescription of male and female, and neotype designation. Zootaxa, 3178 (1), 22–32. https://doi.org/10.11646/zootaxa.3178.1.2
- Bernt, M., Donath, A., Jühling, F., Externbrink, F., Florentz, C., Fritzsch, G., Pütz, J., Middendorf, M. & Stadler, P.F. (2013) MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics & Evolution, 69, 313–319. https://doi.org/10.1016/j.ympev.2012.08.023
- Burger, T.D., Shao, R., Labruna, M.B. & Barker, S.C. (2014a) Molecular phylogeny of soft ticks (Ixodida: Argasidae) inferred from mitochondrial genome and nuclear rRNA sequences. Ticks & Tick-borne Diseases, 5, 195–207. https://doi.org/10.1016/j.ttbdis.2013.10.009
- Camicas, J-L., Hervy, J.P., Adam, F. & Morel, P-C. (1998) The Ticks of the World. Nomenclature, described stages, hosts, distribution (Acarida: Ixodida). Orstom, Paris.
- Chitimia-Dobler, L., Mans, B.J., Handschuh, S. & Dunlop, J.A. (2022) A remarkable assemblage of ticks from mid-Cretaceous Burmese amber. Parasitology, 149, 1–36. https://doi.org/10.1017/S0031182022000269
- Chitimia-Dobler, L., Dunlop, J.A., Pfeffer, T., Würzinger, F., Handschuh, S. & Mans, B.J. (2023) Hard ticks in Burmese amber with Australasian affinities. Parasitology, 150, 157–171. https://doi.org/10.1017/S0031182022001585
- Chitimia-Dobler, L., Handschuh, S., Dunlop, J.A., Pienaar, R. & Mans, B.J. (2025) Nuttalliellidae in Burmese amber: implications for tick evolution. Parasitology, 151, 891–907. https://doi.org/10.1017/S0031182024000477
- Clifford, C.M., Kohls, G.M. & Sonenshine, D.E. (1964) The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). I. The genera and subgenera. Annals of the Entomological Society of America, 57, 429–437. https://doi.org/10.1093/aesa/57.4.429
- Doube, B.M. (1975a) Two races of the kangaroo tick Ornithodoros (Pavlovskyella) gurneyi Warburton. Journal of the Australian Entomological Society, 14, 329–332. https://doi.org/10.1111/j.1440-6055.1975.tb02048.x
- Doube, B.M. (1975b) The biology of the kangaroo tick, Ornithodoros (Pavlovskyella) gurneyi Warburton (Acarina: Argasidae), in the laboratory. Journal of Medical Entomology, 12, 240–243. https://doi.org/10.1093/jmedent/12.2.240
- Estrada-Peña, A. & de la Fuente, J. (2018) The fossil record and the origin of ticks revisited. Experimental & Applied Acarology, 75, 255–261. https://doi.org/10.1007/s10493-018-0261-z
- Fearnhead, E.A. (1962) Investigation on the bionomics of Ornithodoros zumpti Heisch et Guggisberg (Ixodoidea: Argasidae). Zeitschrift für Parasitenkunde, 22, 114–117. https://doi.org/10.1007/BF00329209
- Guglielmone, A.A., Robbins, R.G, Apanaskevich, D.A., Petney, T.N., Estrada-Peña, A. & Horak, I.G. (2014) The Hard Ticks of the World. (Acari: Ixodida: Ixodidae). Springer, Dordrecht, XIII + 738 pp. https://doi.org/10.1007/978-94-007-7497-1
- Hoogstraal, H. & Kohls, G.M. (1966) Ornithodoros (Pavlovskyella) macmillani, new species (Ixodoidea: Argasidae), a nest parasite of Australian cockatoos (Kakatoe roseicapilla). Annals of the Entomological Society of America, 59, 86–92. https://doi.org/10.1093/aesa/59.1.86
- Hoogstraal, H. (1985) Argasid and nuttalliellid ticks as parasites and vectors. Advances in Parasitology, 24, 135–238. https://doi.org/10.1016/s0065-308x(08)60563-1
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology & Evolution, 30, 772–780. https://doi.org/10.1093/molbev/mst010
- Klompen, J.S.H. & Oliver, J.H. (1993) Systematic relationships in the soft ticks (Acari: Ixodida: Argasidae). Systematic Entomology, 18, 313–331. https://doi.org/10.1111/j.1365-3113.1993.tb00669.x
- Klompen, H. & Grimaldi, D. (2001) First Mesozoic record of a parasitiform mite: a larval argasid tick in Cretaceous amber (Acari: Ixodida: Argasidae). Annals of the Entomological Society of America, 94, 10–15. https://doi.org/10.1603/0013-8746(2001)094[0010:FMROAP]2.0.CO;2
- Kneubehl, A.R., Muñoz-Leal, S., Filatov, S., de Klerk, D.G., Pienaar, R., Lohmeyer, K.H., Bermúdez, S.E., Suriyamongkol, T., Mali, I., Kanduma, E., Latif, A.A., Sarih, M., Bouattour, A., de León, A.A.P., Teel, P.D., Labruna, M.B., Mans, B.J. & Lopez, J.E. (2022) Amplification and sequencing of entire tick mitochondrial genomes for a phylogenomic analysis. Scientific Reports, 12, 19310. https://doi.org/10.1038/s41598-022-23393-5
- Lartillot, N., Lepage, T. & Blanquart, S. (2009) PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics, 25, 2286–2288. https://doi.org/10.1093/bioinformatics/btp368
- Laslett, D. & Canbäck, B. (2008) ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics, 24, 172–175. https://doi.org/10.1093/bioinformatics/btm573
- Mans, B.J. (2023) Paradigms in tick evolution. Trends in Parasitology, 39, 475–486. https://doi.org/10.1016/j.pt.2023.03.011
- Mans, B.J., de Klerk, D., Pienaar, R. & Latif, A.A. (2011) Nuttalliella namaqua: a living fossil and closest relative to the ancestral tick lineage: implications for the evolution of blood-feeding in ticks. PLoS One, 6, e23675. https://doi.org/10.1371/journal.pone.0023675
- Mans, B.J., de Klerk, D., Pienaar, R., de Castro, M.H. & Latif, A.A. (2012) The mitochondrial genomes of Nuttalliella namaqua (Ixodoidea: Nuttalliellidae) and Argas africolumbae (Ixodoidae: Argasidae): estimation of divergence dates for the major tick lineages and reconstruction of ancestral blood-feeding characters. PLoS One, 7, e49461. https://doi.org/10.1371/journal.pone.0049461
- Mans, B.J., de Klerk, D., Pienaar, R., de Castro, M.H. & Latif, A.A. (2015) Next-generation sequencing as means to retrieve tick systematic markers, with the focus on Nuttalliella namaqua (Ixodoidea: Nuttalliellidae). Ticks & Tick Borne Diseases, 6, 450–462. https://doi.org/10.1016/j.ttbdis.2015.03.013
- Mans, B.J., Featherston, J., Kvas, M., Pillay, K.A., de Klerk, D.G., Pienaar, R., de Castro, M.H., Schwan, T.G., Lopez, J.E., Teel, P., Pérez de León, A.A., Sonenshine, D.E., Egekwu, N.I., Bakkes, D.K., Heyne, H., Kanduma, E.G., Nyangiwe, N., Bouattour, A. & Latif, A.A. (2019) Argasid and ixodid systematics: Implications for soft tick evolution and systematics, with a new argasid species list. Ticks & Tick Borne Diseases, 10, 219–240. https://doi.org/10.1016/j.ttbdis.2018.09.010
- Mans, B.J., Kelava, S., Pienaar, R., Featherston, J., de Castro, M.H., Quetglas, J., Reeves, W.K., Durden, L.A., Miller, M.M., Laverty, T.M., Shao, R., Takano, A., Kawabata, H., Moustafa, M.A.M., Nakao, R., Matsuno, K., Greay, T.L., Evasco, K.L., Barker, D. & Barker, S.C. (2021) Nuclear (18S-28S rRNA) and mitochondrial genome markers of Carios (Carios) vespertilionis (Argasidae) support Carios Latreille, 1796 as a lineage embedded in the Ornithodorinae: re-classification of the Carios sensu Klompen and Oliver (1993) clade into its respective subgenera. Ticks & Tick Borne Diseases, 12, 101688. https://doi.org/10.1016/j.ttbdis.2021.101688
- McLoughlin, S. (2001) The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany, 49, 271–300. https://doi.org/10.1071/BT00023
- Minh, B.Q., Schmidt, H.A., Chernomor, O., Schrempf, D., Woodhams, M.D., von Haeseler, A. & Lanfear, R. (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology & Evolution, 37, 1530–1534. https://doi.org/10.1093/molbev/msaa015
- Muñoz-Leal, S., Venzal, J.M., Kneubehl, A.R., Lopez, J.E., Martins, T.F. & Labruna, M.B. (2023) Description of a new Pavlovskyella species (Acari: Argasidae) from Chile. Journal of Medical Entomology, 60, 968–977. https://doi.org/10.1093/jme/tjad091
- Petney, T.N., Andrews, R.H., McDiarmid, L.A. & Dixon, B.R. (2004) Argas persicus sensu stricto does occur in Australia. Parasitology Research, 93, 296–299. https://doi.org/10.1007/s00436-004-1141-5
- Poinar Jr, G.O. (1995) First fossil of soft ticks, Ornithodoros antiquus n. sp. (Acari: Argasidae) in Dominician amber with evidence of their mammalian host. Experentia, 51, 384–387.
- Pospelova-Shtrom, M.V. (1969) On the system of classification of ticks of the family Argasidae Can., 1890. Acarologia, 11, 1–22.
- Ribeiro, C.C., Faccini, J.L., Cançado, P.H., Piranda, E.M., Barros-Battesti, D.M. & Leite, R.C. (2013) Life cycle of Ornithodoros rostratus (Acari: Argasidae) under experimental conditions and comments on the host-parasite relationship in the Pantanal wetland region, Brazil. Experimental & Applied Acarology, 61, 139–146. https://doi.org/10.1007/s10493-013-9669-7
- Seton, M., Müller, R.D., Zahirovic, S., Gaina, C., Torsvik, T., Shepard, G., Talsma, A., Gurnis, M., Turner, M., Maus, S. & Chandler, M. (2012) Global continental and ocean basin reconstructions since 200 Ma. Earth-Science Reviews, 113, 212–270. https://doi.org/10.1016/j.earscirev.2012.03.002
- Warburton, C. (1926) On three new species of ticks (Arachnida, Ixodoidea), Ornithodorus gurneyi, Ixodes arvicolae and Haemaphysalis mjöbergi. Parasitology, 18, 55–58. https://doi.org/10.1017/S0031182000004972