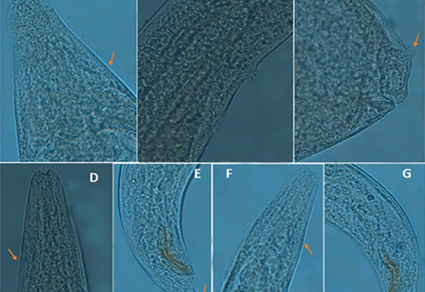
Abstract
A population of entomopathogenic nematodes, belonging to the Feltiae-clade and labelled J13, was discovered in the agricultural soils of the hilly regions of the Union territory of Jammu and Kashmir, India. Based on morphological, morphometric, and molecular analyses, the nematodes were identified as Steinernema feltiae. The J13 nematode isolate was tested in a laboratory assay for its pathogenicity against six major pests of vegetable crops: Pieris brassicae, Plutella xylostella, Helicoverpa armigera, Agrotis iplison, Trichoplusia ni, and Exelastis atomosa. The morphology of the isolated nematode closely matched the original description, except for the adult females, which had prominent epiptygmata instead of the weakly developed, double-flapped epiptygmata described in the original report. Analysis of the internal transcribed spacer and large subunit rRNA data from the J13 nematodes showed 100% similarity to sequences of the type population, indicating that they are conspecific. The virulence assays revealed that the nematode caused 100% mortality in the tested insect pests within 48–72 hours, even at the lowest concentration of 50 infective juveniles per insect. The calculated median lethal concentration varied among the pests, with the lowest number of infective juveniles needed to achieve 50% larval killing being 117 for P. xylostella, 181.74 for P. brassicae, 226.35 for H. armigera, and 202.07 for T. ni at 24 hours post-inoculation. These findings suggest that S. feltiae isolated during the present investigation, may be a viable option for the biocontrol of these insect pests in Kashmir valley, India.
References
- Aasha, Chaubey, A.K. & Bhat, A.H. (2019) Notes on Steinernema abbasi (Rhabditida: Steinernematidae) strains and virulence tests against lepidopteran and coleopteran pests. Journal of Entomology and Zoology Studies, 7, 954–964. https://doi.org/10.1163/15685411-00002956
- Abate, B.A., Malan, A.P., Tiedt, L.R., Wingfield, M.J., Slippers, B. & Hurley BP (2016) Steinernema fabii n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology, 18, 235–255.
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990) Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
- Askary, T.H. & Abd-Elgawad, M.M.M. (2021) Opportunities and challenges of entomopathogenic nematodes as biocontrol agents in their tripartite interaction. Egyptian Journal of Biological Pest Control, 31, 42. https://doi.org/10.1186/s41938-021-00391-9
- Askary, T.H. & Ahmad, M.J. (2021) Biocidal efficacy of some native isolates of entomopathogenic nematodes against oriental armyworm, Mythimna separata Walker (Lepidoptera: Noctuidae). Indian Journal of Nematology, 51, 67–73. https://doi.org/10.5958/0974-4444.202L0y010.X
- Askary, T.H. & Ahmad, M.J. (2020) Efficacy of entomopathogenic nematodes against the cabbage butterfly (Pieris brassicae (L.) (Lepidoptera: Pieridae) infesting cabbage under field conditions. Egyptian Journal of Biological Pest Control, 30, 39. https://doi.org/10.1186/s41938-020-00243-y
- Askary, T.H., Bhat, A.H., Machado, R.A.R., Ahmad, M.J., Abd-Elgawad, M.M.M., Khan, A.A. & Gani, M. (2023) Virulence and reproductive potential of Indian entomopathogenic nematodes against the larvae of the rice meal moth. Archives of Phytopathology and Plant Protection, 56, 1–13. https://doi.org/10.1080/03235408.2022.2161293
- Askary, T.H., Bhat, A.H., Ahmad, M.J., Chaubey, A.K. & Spiridonov, S.E. (2020) Steinernema feltiae (Rhabditida: Steinernematidae) from hilly areas of Kashmir valley, India with a note on its geographical distribution. Russian Journal of Nematology, 28, 99–106.
- Askary, T.H., Ahmad M.J., Wani, A.R., Mohiddin, S. & Sofi, M.A. (2018) Behavioural ecology of entomopathogenic nematodes, Steinernema and Heterorhabditis for Insect biocontrol. In: Lichtfouse, E. (Ed.), Sustainable Agriculture Reviews. Springer, Cham, pp. 425–441. https://doi.org/10.1007/978-3-319-94232-2_8
- Askary, T.H. (2010) Nematodes as biocontrol agents. In: Lichtfouse, E. (Ed.), Sociology, Organic Farming, Climate Change and Soil Science. Springer, Dordrecht, pp. 347–378. https://doi.org/10.1007/978-90-481-3333-8_13
- Baldwin, J.G., Nadler, S.A. & Adams, B. (2004) Evolution of plant parasitism among nematodes. Annual Review of Phytopathology, 42, 83–105. https://doi.org/10.1146/annurev.phyto.42.012204.130804
- Bedding, R.A. & Akhurst, R.J. (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica, 21, 109–110. https://doi.org/10.1163/187529275X00419
- Bhat, A.H., Chaubey, A.K. & Askary, T.H. (2020) Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egyptian Journal of Biological Pest Control, 30, 31. https://doi.org/10.1186/s41938-020-0212-y
- Bhat, A.H., Askary, T.H., Ahmad, M.J., Bhargava, S., Rana, A. & Chaubey, A.K. (2019) Description of Heterorhabditis bacteriophora (Nematoda: Heterorhabditidae) isolated from hilly areas of Kashmir valley. Egyptian Journal of Biological Pest Control, 29, 96. https://doi.org/10.1186/s41938-019-0197-6
- Bhat, A.H., Chaubey, A.K., Hartmann, J. & Půža, V. (2021a) Notes on the morphology, bionomics, distribution and efficacy of Steinernema siamkayai (Rhabditida: Steinernematidae) from Western Uttar Pradesh, India. Nematology, 23, 817–836. https://doi.org/10.1163/15685411-bja10079
- Bhat, A.H., Rana, A., Chaubey, A.K., Shokoohi, E. & Machado, R.A.R. (2021b) Characterisation of Steinernema abbasi (Rhabditida: Steinernematidae) isolated from Indian agricultural soils and their efficacy against insect pests. Biocontrol Science and Technology, 31, 1027–1051. https://doi.org/10.1080/09583157.2021.1917514
- Bhat, A.H., Chaubey, A.K., Shokoohi, E. & Machado, R.A.R. (2021c) Molecular and phenotypic characterization of Heterorhabditis indica (Nematoda: Rhabditida) nematodes isolated during a survey of agricultural soils in Western Uttar Pradesh, India. Acta Parasitologica, 66, 236–252. https://doi.org/10.1007/s11686-020-00279-y
- Bhat, A.H., Chaubey, A.K. & Půža, V. (2018) The first report of Xenorhabdus indica from Steinernema pakistanense: co-phylogenetic study suggests co-speciation between X. indica and its steinernematid nematodes. Journal of Helminthology, 92, 81–90. https://doi.org/10.1017/S0022149X17001171
- Bhat, A.H., Chaubey, A.K., Shokoohi, E. & Mashela, P.W. (2019) Study of Steinernema hermaphroditum (Nematoda, Rhabditida), from the West Uttar Pradesh, India. Acta Parasitologica, 64, 720–737. https://doi.org/10.2478/s11686-019-00061-9
- Bhat, A.H., Gautum, S., Rana, A., Chaubey, A.K., Abolafia, J. & Půža, V. (2022a) Morphological, morphometrical and molecular characterization of Oscheius siddiqii Tabassum and Shahina, 2010 (Rhabditida, Rhabditidae) from India with its taxonomic consequences for the subgenus Oscheius Andrássy, 1976. Biology, 10, 1239. https://doi.org/10.3390/biology10121239
- Bhat, A.H., Istkhar, Chaubey, A.K., Půža, V. & San-Blas, E. (2017) First report and comparative study of Steinernema surkhetense (Rhabditida: Steinernematidae) and its symbiont bacteria from sub-continental India. Journal of Nematology, 49, 92–102. https://doi.org/10.21307/jofnem-2017-049
- Bhat, A.H., Ameni, L., Abolafia, J., Machado, R.A.R. & Kallel, S. (2022b) Comparative morphological and molecular analyses of Acrobeloides bodenheimeri and A. tricornis Cobb, 1924 (Rhabditida, Cephalobidae) from Tunisia. Nematology, 25, 207–226. https://doi.org/10.1163/15685411-bja10215
- Bhat, A.H., Machado, R.A.R., Abolafia, J., Askary, T.H., Půža, V., Ruiz-Cuenca, A.N., Rana, A., Sayed, S. & Al-Shuraym, L.A. (2023) Multigene sequence based and phenotypic characterization reveals the occurrence of a novel entomopathogenic nematode species, Steinernema anantnagense n. sp. Journal of Nematology, 55, 20230029. https://doi.org/10.2478/jofnem-2023-0029
- Boff, M.I.C., Wiegers, G.L. & Smits, P.H. (2000) The influence of storage temperature and time on infectivity and reproduction of Heterorhabditis megidis (strain NLH-E87. 3). International Organization for Biological and Integrated Control. West Palaearctic Regional Section Bulletin, 23, 53–60.
- Caroli, L., Glazer, I. & Gaugler, R. (1996) Entomopathogenic nematode infectivity assay: Comparision of penetration rate into different hosts. Biocontrol Science and Technology, 6, 227–233. https://doi.org/10.1080/09583159650039412
- Chevenet, F., Brun, C., Bañuls, A.L., Jacq, B. & Christen, R. (2006) TreeDyn: Towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics, 7 (1), 439. https://doi.org/10.1186/1471-2105-7-439
- Courtney, W.D., Polley, D. & Miller, V.L. (1955) TAF, an improved fixative in nematode technique. Plant Disease Reports, 39, 570–571.
- Edgar, R.C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32 (5), 1792–1797. https://doi.org/10.1093/nar/gkh340
- Filipjev, I.N. (1934) Eine neue Art der Gattung Neoaplectana Steiner nebst Bermerkungen über die systematische Stellung der letzteren. Parazitologichesky. Miscellanea Nematologica, 4, 229–240.
- Forschler, B.T. & Nordin, G.L. (1988) Comparative pathogenicity of selected entomogenous to the hardwood borers, Prionoxystus robiniae (Lepidoptera: Cosidae) and Megacyllene robiniae (Coleoptera: Cerambicidae). Journal of Inverbrate Pathology, 52, 343–347. https://doi.org/10.1016/0022-2011(88)90144-9
- Gaugler, R. (1988) Ecological considerations in the biological control of soil-inhabiting insect pests with entomopathogenic nematodes. Agriculture Ecosystems and Environment, 24, 351–360. https://doi.org/10.1016/0167-8809(88)90078-3
- Glazer, I. & Navon, A. (1990) Activity and persistence of entomoparasitic nematodes tested against Heliothis armigera (Lepidoptera: Noctuidae). Journal of Economic Entomology, 83, 1795–1800. https://doi.org/10.1093/jee/83.5.1795
- Hall, T.A. (1999) BioEdit: a user–friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
- Harris, N.C., Coonan, T.J., King, J.L. & Dunn, R.R. (2013) Endemism in host-parasite interactions among island populations of an endangered species. Diversity and Distrbutions, 19, 377–380. https://doi.org/10.1111/ddi.12016
- Hunt, D.J. & Nguyen, K.B. (2016) Advances in Entomoparasitic Nematode Taxonomy and Phylogeny. In: Hunt, D.J. & Perry, R.N. (Eds.), Nematology Monographs and Perspectives. Vol. 12. Brill, Leiden, pp. 1–438. https://doi.org/10.1163/9789004285347
- Janardhan, H.N. (2022) Diversity and distribution of entomopathogenic nematodes in vegetable growing areas of Baramulla district in Kashmir. M.Sc. (Ag.) Thesis, Division of Entomology, Faculty of Agriculture, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Srinagar, Jammu and Kashmir. [unknown pagination]
- Kajol, Y., Bhat, A.H., Aasha, R. & Chaubey, A.K. (2020) Biochemical and molecular characterization of associated Photorhabdus symbiont of Indian strain of Heterorhabditis indica and its efficacy. Pakistan Journal of Nematology, 38, 15–24. https://doi.org/10.18681/pjn.v38.i01.p15-24
- Kaya, H.K. & Gaugler, R. (1993) Entomopathogenic nematodes. Annual Review of Entomology, 38, 181–206.
- Khan, M.R., Khan, U. & Askary, T.H. (2007) Occurrence of Steinernema masoodi in Aligarh and its pathogenicity against six economically important insect pests. Indian Journal of Nematology, 37, 215–216. https://doi.org/10.1146/annurev.en.38.010193.001145
- Kim, J.B., Park, J.D. & Kim, C.S. (1995) The distribution and pathogenicity of entomogenous nematodes in forest soil in Korea. FRI Journal of Forest Science, Seoul, 51, 74–79.
- Kimura, M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16 (2), 111–120. https://doi.org/10.1007/BF01731581
- Lalramliana & Yadav, A.K. (2009) Laboratory evaluation of the pathogenicity of three entomopathogenic nematodes against larvae of cabbage butterfly, Pieris brassicae Linnaeus (Lepidoptera: Pieridae). Science Vision, 9, 166–173.
- Lewis, E.E., Gaugler, R. & Harrison, R. (1992) Entomopathogenic nematode host finding: response to host contact cues by cruise and ambush foragers. Parasitology, 105, 309–319. https://doi.org/10.1017/S0031182000074230
- Letunic, I. & Bork, P. (2016) Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research, 44 (Web Server Issue), W242–W245. https://doi.org/10.1093/nar/gkw290
- Loulo, A., Maristella, M., Sara, C., Bhat, A.H., Maurizio, B., Machado, R.A.R. & Kallel, S. (2023) Entomopathogenic potential of bacteria associated with soil-borne nematodes and insect immune responses to their infection. PLoS ONE, 18, e0280675. https://doi.org/10.1371/journal.pone.0280675
- Loulou, A., Guerfali, M.M., Muller, A., Bhat, A.H., Abolafia, J., Machado, R.A.R. & Kallel, S. (2022) Potential of Oscheius tipulae nematodes as biological control agents against Ceratitis capitate. PLoS ONE, 17 (6), e0269106. https://doi.org/10.1371/journal.pone.0269106
- Nadler, S.A., De Ley, P., Mundo-Ocampo, M., Smythe, A.B., Stock, S.P., Bumbarger, D., Adams, B.J., De Ley, T., Holovachov, I.O. & Baldwin, J.G. (2006) Phylogeny of Cephalobina (Nematoda): molecular evidence for recurrent evolution of probolae and incongruence with traditional classifications. Molecular Phylogenetics and Evolution, 40, 696–711. https://doi.org/10.1016/j.ympev.2006.04.005
- Nielsen, O., Skovgaard, I.M. & Philipsen, H. (2004) Estimating the incidence of entomopathogenic nematodes in soil by the use of bait insects. Nematology, 6, 891–900. https://doi.org/10.1163/1568541044038687
- Machado, R.A.R., Bhat, A.H., Abolafia, J., Shokoohi, E., Fallet, P., Turlings, T.C.J., Tarasco, E., Půža, V., Kajuga, J., Yan, X. & Toepfer, S. (2022) Steinernema africanum n. sp. (Rhabditida, Steinernematidae), a new entomopathogenic nematode species isolated in the Republic of Rwanda. Journal of Nematology, 54, e2022. https://doi.org/10.2478/jofnem-2022-0049
- Machado, R.A.R., Bhat, A.H., Fallet, P., Turlings, T.C.J., Kajuga, J., Yan, X. & Toepfer, S. (2023a) Xenorhabdus bovienii subsp. africana subsp. nov., isolated from Steinernema africanum entomopathogenic nematodes. International Journal of Systematic and Evolutionary Microbiology, 73 (4). https://doi.org/10.1099/ijsem.0.005795
- Machado, R.A.R., Bhat, A.H., Castaneda-Alvarez, C., Askary, T.H., Půža, V., Pagès, S., & Abolafia, J. (2023b) Xenorhabdus aichiensis sp. nov., X. anantnagensis sp. nov., and X. yunnanensis sp. nov., isolated from Steinernema entomopathogenic nematodes. Current Microbiology, 80, 300. https://doi.org/10.1007/s00284-023-03373-2
- Rahoo, A.M., Mukhtar, T., Jakhar, A.M. & Rahoo, R.K. (2018) Inoculum dose and exposure periods affect recovery of Steinernema feltiae and Heterorhabditis bacteriophora from Tenebrio molitor. Pakistan Journal of Zoology, 50, 983–987. https://doi.org/10.17582/journal.pjz/2018.50.3.983.987
- Rana, A., Bhat, A.H., Shokoohi, E. & Machado, R.A.R. (2020) Morphological and molecular characterization Heterorhabditis bacteriophora nematodes isolated from Indian agricultural soils and their biocontrol potential. Zootaxa, 4878 (1), 77–102. https://doi.org/10.11646/zootaxa.4878.1.3
- Razak, N. & Ahmad, I. (2020) Diversity of Insects Infesting medicinal and aromatic plants in the Kashmir Valley. In: Dar, G. & Khuroo, A. (Eds.), Biodiversity of the Himalaya: Jammu and Kashmir State. Topics in Biodiversity and Conservation. Vol. 18. Springer, Singapore, pp. 801–820. https://doi.org/10.1007/978-981-32-9174-4_30
- Seinhorst, J.W. (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin G. Nematologica, 4, 67–69. https://doi.org/10.1163/187529259X00381
- Spiridonov, S.E., Reid, A.P., Podrucka, K., Subbotin, S.A. & Moens, M. (2004) Phylogenetic relationships within the genus Steinernema (Nematoda: Rhabditida) as inferred from analyses of sequences of the ITS1-5.8S-ITS2 region of rDNA and morphological features, Nematology, 6, 547–566. https://doi.org/10.1163/1568541042665304
- Sunanda, B.S., Jeyakumar, P. & Jacob, V.V. (2014) Bioefficacy of different formulations of entomopathogenic nematode Steinernema carpocapsae against diamond back moth (Plutella xylostella) infesting cabbage (Brassica oleracea var. capitata). Journal of Biopesticides, 7, 210–215.
- Tamura, K., Stecher, G. & Kumar, S. (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution, 38 (7), 3022–3027. https://doi.org/10.1093/molbev/msab120
- Thanwisai, A., Tandhavanant, S., Saiprom, N., Waterfield, N.R., Ke Long, P., Bode, H.B., Peacock, S.J. & Chantratita, N. (2012) Diversity of Xenorhabdus and Photorhabdus spp. and their symbiotic entomopathogenic nematodes from Thailand. PLoS One, 7 (9), e43835. https://doi.org/10.1371/journal.pone.0043835
- Travassos, L. (1927) Sobre o genero Oxysomatium. Boletim Biologico, Sao Paulo, 5, 20–21.
- Vrain, T.C., Wakarchuk, D.A., Lévesque, A. & Hamilton, R.I. (1992) Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundamentals and Applied Nematology, 15, 563–73.
- White, G.F. (1927) A method for obtaining infective nematode larvae from cultures. Science, 66, 302–303. https://doi.org/10.1126/science.66.1709.302-a
- Wouts, W.M., Mráček, Z., Gerdin, S. & Bedding, R.A. (1982) Neoaplectana Steiner, 1929 a junior synonym of Steinernema Travassos, 1927 (Nematoda: Rhabditida). Systematic Parasitology, 4, 147–154. https://doi.org/10.1007/BF00018998
- Yadav, K., Bhat, A.H., Chaubey, A.K., Machado, R.A.R. & Abolafia, J. (2023) Redescription and molecular characterization of Panagrolaimus labiatus (Kreis, 1929) Andrássy, 1960 (Rhabditida, Panagrolaimidae) from India and proposal of P. burdwanensis Chaturvedi and Khera, 1979 as a junior synonym of P. labiatus. Nematology, 25, 151–168. https://doi.org/10.1163/15685411-bja10211
- Zervos, S., Johnson, S.C. & Webster, J.M. (1991) Effect of temperature and inoculum size on reproduction and development of Heterorhabditis heliothidis and Steinernema glaseri (Nematoda: Rhabdittoidea) in Galleria mellonella. Canadian Journal of Zoology, 69, 1261–1264. https://doi.org/10.1139/z91-177