Abstract
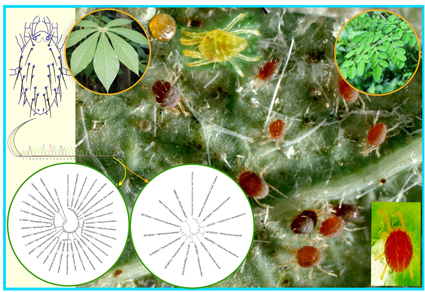
Host- and habitat-induced morphological shape and size variations are common in phytophagous and parasitic taxa. Several integrated morphological and molecular techniques have been commonly used to understand host-induced morpho-cryptic species forms. Compared to other arthropods, cryptic speciation was more common in Acari. This study focused on the host-specific morphological cryptic shape and size variations of Tetranychus neocaledonicus, collected from moringa and cassava hosts. We used geometric morphometric analysis to uncover the shape and size of inter-and intra-spider mite populations, and discovered that host-specific shape and size variations existed in spider mites regardless of sex. Interestingly, there was no phylogenetic signal in spider mites, implying that the morpho-cryptic speciation of T. neocaledonicus is solely based on the host-induced selection. The molecular clock hypothesis was accepted in our CO1 and 18s rRNA phylogeny analyses, and spider mites collected from both hosts were genetically less diverse. We conclude that T. neocaledonicus exhibited morphologically detectable cryptic population diversity in each host but that these populations are evolutionarily young form. Apart from these host-induced variations, we also monitored the impact of the clearing agent (lactic acid) on the shape and size of T. neocaledonicus; from this study, we proved that the clearing agent significantly alters the taxonomically important morphological traits of spider mites irrespective of the mites’ sex, as confirmed by multivariate statistical analysis. This is the first study report to investigated the host-induced morphological variations of spider mites and the impact of a clearing agent.
References
- Abdallah, A.A., Zhang, Z.Q., Masters, G.J. & Mcneill, S. (2001) Euseius finlandicus (Acari: Phytoseiidae) as a potential biocontrol agent against Tetranychus urticae (Acari: Tetranychidae): life history and feeding habits on three different types of food. Experimental and Applied Acarology, 25, 833–847. https://doi.org/10.1023/A:1020431531446
- Anand, P.P. & Ramani, N. (2021a) Dynamics of limited neoplastic growth on Pongamia pinnata (L.) (Fabaceae) leaf, induced by Aceria pongamiae (Acari: Eriophyidae). BMC Plant Biology, 21 (1). [published online] https://doi.org/10.1186/s12870-020-02777-7
- Anand, P.P. & Ramani, N. (2021b) Enzymatic characterization of the saliva of the eriophyid mite, Aceria pongamiae Keifer 1966 (Acari: Eriophyidae) and the bacterial endobiome of the galls induced on Pongamia pinnata (L.) Pierre. The Science of Nature, 108 (33). [published online] https://doi.org/10.1007/s00114-021-01743-z
- Anand, P.P., Seena, S., Jinsha-Peter. & Shibu Vardhanan, Y. (2022) Detection of geographical specific plasticity and the effect of natural selection pressure on the wing size and shape of Bactrocera dorsalis (Diptera: Tephritidae). Biologia, 77 (4), 1347–1371. https://doi.org/10.1007/s11756-022-01059-x
- Anand, P. P., Seena, S., Girish Kumar, P. & Y. Shibu Vardhanan. (2023). Species morphospace boundary revisited through wing phenotypic variations of Antodynerus species (Hymenoptera: Vespidae: Eumeninae) from the Indian subcontinent. Frontiers in Ecology and Evolution. 10, 965577.
- https://doi.org/10.3389/fevo.2022.965577
- Arbiv, A., Khokhlova, I.S., Ovadia, O., Novoplansky, A. & Krasnov, B. R. (2012) Use it or lose it: reproductive implications of ecological specialization in a hematophagous ectoparasite. Journal of Evolutionary Biology, 25, 1140–1148. https://doi.org/10.1111/j.1420-9101.2012.02499.x
- Arambourou, H., Beisel, J.N., Branchu, P. & Debat, V. (2012) Patterns of fluctuating asymmetry and shape variation in Chironomus riparius (Diptera: Chironomidae) exposed to nonylphenol or lead. Plos One, 7, e48844. https://doi.org/10.1371/journal.pone.0048844
- Armstrong, K.F. & Ball, S.L. (2005) DNA barcodes for biosecurity: invasive species identification. Philosophical Transactions of the Royal Society B, 360, 1813–1823. https://doi.org/10.1098/rstb.2005.1713
- Baker, E.W. & Tuttle, D.M. (1994) A guide to the spider mites (Tetranychidae) of the United States. Vol. 1. Indira Publishing House, West Bloomfield, Michigan, 347 pp.
- Baran, S., Altum, A., Ayyildiz, N. & Kence, A. (2011) Morphometric analysis of oppiid mites (Acari, Oribatida) collected from Turkey. Experimental and Applied Acarology, 54, 411–420. https://doi.org/10.1007/s10493-011-9448-2
- Becerra, J.M. & Valdecasas, A.G. (2004) Landmark superimposition for taxonomic identification. Biological Journal of Linnean Society, 81, 267–274. https://doi.org/10.1111/j.1095-8312.2003.00286.x
- Bickford, D., Lohman, D.J., Sodhi, N.S., Ng, P.K.L., Meier, R., Winkler, K., Ingram, K.K. & Das, I. (2007) Cryptic species as a window on diversity and conservation. Trends in Ecology and Evolution, 22, 148–155.
- https://doi.org/10.1016/j.tree.2006.11.004
- Blahnik, R.J., Holzenthal, R.W. & Prather, A. (2007) The lactic acid method for clearing Trichoptera genitalia. In: Bueno-Soria, J., Barba-Alvarez, R. & Armitage, B. (Eds.), Proceedings of the XIIth International Symposium on Trichoptera. Caddis press, Columbus, Ohio, pp. 9–14.
- Blair, C.P., Abrahamson, W.G., Jackman, J.A. & Tyrrell, L. (2005) Cryptic speciation and host-race formation in a purportedly generalist tumbling flower beetle. Evolution, 59, 304–316. https://doi.org.10.1554/03-705
- Bookstein, F.L. (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge/New York/Port Chester/Melbourne/Sydney, XVIII + 435 pp.
- Brooks, D.R. & McLennan, D.A. (1993) Macroevolutionary patterns of morphological diversification among parasitic flatworms (Platyhelminthes, Cercomeria). Evolution, 47, 495–509. https://doi.org/10.1111/j.1558-5646.1993.tb02109.x
- Burger, T.D., Shao, R. & Barker, S.C. (2014) Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Molecular Phylogenetics and Evolution, 76, 241–253. https://doi.org/10.1016/j.ympev.2014.03.017
- Butler, J.L. (1992) Collection and preservation of materials for otolith analysis. In: Stevenson, D.K. & Campana, S.E. (Eds.), Otolith structure examination and analysis. Canadian Journal of Fisheries and Aquatic Science, 117, pp. 13–17.
- Carew, M., Schiffer, M., Umina, P., Weeks, A. & Hoffmann, A. A. (2009) Molecular markers indicate that the wheat curl mite, Aceria tosichella Keifer, may represents a species complex in Australia. Bulletin of Entomological Research, 99, 479–286. https://doi.org/10.1017/S0007485308006512
- Clarke, F.C. & Pretorius, E. (2005) A comparison of geometric morphometric analyses and cross-breeding as methods to determine relatedness in three Amblyomma species (Acari: Ixodidae). International Journal of Acarology, 31, 393–405. https://doi.org/10.1080/01647950508683681
- Cooke, G.M., Chao, N.L. & Beheregaray, L.B. (2012) Five cryptic species in the Amazonian catfish Centromochlus existimatus identified based on biogeography prediction and genetic data. PLos ONE, 7 (11), e48800.
- https://doi.org/10.1371/journal.pone.0048800
- De Meeus, T. (2000) Adaptive diversity, specialization, habitat preference and parasites. In: Poulin, R., Morand, S. & Skorping, A. (Eds.), Evolutionary biology of host-parasite relationships: theory meets reality. Elsevier, Amsterdam, pp. 27–42.
- Deunff, J., Walter, G., Bellido, A. & Volleth, M. (2004) Description of a cryptic species, Spinturnix bechsteini n. sp. (Acari, Mesostigmata, Spinturnicidae), parasite of Myotis bechsteinii (Kuhl, 1817) (Chiroptera, Vespertilionidae) by using ecoethology of host bats and statistical methods. Journal of Medical Entomology, 41, 826–832. https://doi.org/10.1603/0022-2585-41.5.826
- Dryden, I.L. & Mardia, K.M. (1998) Statistical shape analysis. Wily, New York. [unknown pagination]
- Duso, C., Pasini, M. & Pellegrini, M. (2003) Distribution of the predatory mite Typhlodromus pyri (Acari: Phytoseiidae) on different apple cultivars. Biocontrol Science and Technology, 13, 671–681. https://doi.org/10.1080/09583150310001606264
- Fey, D.P. (1999) Effects of preservation technique on the length of larval fish: methods of correcting estimates and their implication for studying growth rates. Archive of Fishery and Marine Research, 417 (1), 17–29.
- Fey, D.P. (2012) Length adjustment of larval and early-juvenile cod (Gadus morhua) after up to 3 years of preservation in alcohol. Journal of Applied Ichthyology, 28 (4), 665–666. https://doi.org/10.1111/j.1439-0426.2011.01929.x
- Flechtmann, C.H.W. & Knihinicki, D.K. (2002) New species and new records of Tetranychus Dufour from Australia, with a key to the major groups in this genus based on females. Australian Journal of Entomology, 41, 118–127. https://doi.org/10.1046/j.1440-6055.2002.00289.x
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3 (5), 294–299.
- Fruciano, C. (2016) Measurement error in geometric morphometrics. Development Genes and Evolution, 226, 139–158. https://doi.org/10.1007/s00427-016-0537-4
- Futuyma, D.J. & Moreno, G. (1988) The evolution of ecological specialization. Annual Review of Ecology and Systematics, 19, 207–233. https://doi.org/10.1146/annurev.es.19.110188.001231
- Gidaszewski, N.A., Baylac, M. & Klingenberg, C.P. (2009) Evolution of sexual dimorphism of wing in the Drosophila melanogaster sub group. BMC Evolutionary Biology, 9 (110). [published online] https://doi.org/10.1186/1471-2148-9-110
- Gilbert, S.F. (2006) Developmental biology. Sinauer, Sunderland, Massachusetts, USA.
- Giribert, G., Carranza, S., Baguna, J., Riutort, M. & Ribera, C. (1996) First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Molecular Biology and Evolution, 13, 76–84. https://doi.org/10.1093/oxfordjournals.molbev.a025573
- Gould, S.J. (1966) Allometry and size in ontogeny and phylogeny. Biological Reviews, 41, 587–640. https://doi.org/10.1111/j.1469-185x.1966.tb01624.x
- Gumiel, M., Catala, S., Noireau, F., Rojas De Arias, A., Garcia, A. & Dujardin, P. (2003) Wing geometry in Triatoma infestans (Klug) and T. melanosome Martinez, Olmedo & Carcavallo (Hemiptera: Reduviidae). Systematic Entomology, 28 (2), 173–180. https://doi.org/10.1046/j.13365-3113.2003.00206.x
- Heethoff, M., Domes, K., Laumann, M., Maraun, M., Norton, R.A. & Scheu, S. (2007) High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari: Oribatida). Journal of Evolutionary Biology, 20, 392–402. https://doi.org/10.1111/j.1420-9101.2006.01183.x
- Herbert, P.D.N., Ratnasingham, S. & de Waard, R. (2003) Barcoding animal life: cytochrome c oxidase subunit I divergence among closely related species. Proceeding Royal Society of London B, 270, 96–99. https://doi.org/10.1098/rsbi.2003.0025
- Hermission, J., Hansen, T.F. & Wagner, G. P. (2003) Epistasis in polygenic traits and the evolution of genetic architecture under stabilizing selection. The American Naturalist, 161 (5), 708–734. https://doi.org/10.1086/374204
- Jacinaviaus, F.C., Badari, J.C., Ramirez, D.G., Moraes, R.H.P., Onofrio, V.C. & Barros-Battesti, D.M. (2013) Technique for restoration of mite (Acari) preparations in deteriorated Hoyer’s medium. Neotropical Entomology, 42, 328–329.
- https://doi.org/10.1007/s13744-013-0129-1
- Jagersbacher-Baumann, J. (2014) Species differentiation of scutacarid mites (Heterostigmatatina) using multivariate morphometric methods. Experimenal and Applied Acarology, 62, 279–292. https://doi.org/10.1007/s10493-013-9747-x
- Jagersbacher-Baumann, J. & Ebermann, E. (2012) Fungal spore transfer and intraspecific variability of a newly described African soil mite (Heterostigmata, Scutacaridae, Heterodispus). Zoological Anzeiger, 251 (2), 101–114. https://doi.org/10.1016/j.jcz.2011.05.008
- Johanson, K.P., Shreve, S.M. & Smith, V.S. (2012) Repeated adaptive divergence of microhabitat specialization in avian feather lice. BMC Biology, 10, 52. https://doi.org/10.1186/1741-7007-10-52
- Kankare, M., Constanti, S., Van Nouhuys, S. & Shaw, M.R. (2005) Host-specialization by Cotesia wasps (Hymenoptera: Braconidae) parasitizing species-rich Melitaeini (Lepidoptera: Nymphalidae) communities in north-eastern Spain. Biological Journal of Linnean Society, 86 (1), 45–65. https://doi.org/10.1111/j.1095-8312.2005.00523.x
- Kassen, R. (2002) The experimental evolution of specialists, generalists, and the maintenance of diversity. Journal of Evolutionary Biology, 15, 173–190. https://doi.org/10.1046/j.1420-9101.2002.00377.x
- Katzke, J., Barden, P., Dehon, M., Michez, D. & Wappler, T. (2018) Giant ants and their shape: revealing relationships in the genus Titanomyrma with geometric morphometrics. Peer J, 6, e4242. https://doi.org/10.7717/peerj.4242
- Kendall, D.G. (1977) The diffusion of shape, Advances in Applied Probability, 9 (3), 428–430. https://doi.org/10.2307/1426091
- Kerschbaumer, M. & Pfingstl, T. (2021) Testing for phylogenetic signal in claws suggests great influence of ecology on Caribbean intertidal arthropods (Acari, Oribatida). Scientific Report, 11, 4398. https://doi.org/10.1038/s41598-021-83747-3
- Klimov, P.B., Bochkov, A.V. & Oconnor, B.M. (2006) Host specificity and multivariate diagnostics of cryptic species in predacious cheyletid mites of the genus Cheletophyes (Acari: Cheyletidae) associated with large carpenter bees. Biological Journal of Linnean Society, 87, 45–58. https://doi.org/10.1111/j.1095-8312.2006.00554.x
- Klimov, P.B., Lekveishvili, M., Dowling, A.P.G. & Oconnor, B.M. (2004) Multivariate analysis of morphological variations in two cryptic species of Sancassania (Acari: Acaridae) from Csta Rica. Annals of the Entomological Society of America, 97 (2), 322–345. https://doi.org/10.1093/aesa/97.2.322
- Klimov, P.B. & Oconnor, B.M. (2004) Multivariate discrimination among cryptic species of the mite genus Chaetodactylus (Acar: Chaetodactylidae) associated with beed of the genus Lithurgus (Hymenoptera: Megachilidae) in North America. Experimental and Applied Acarology, 33, 157–182. https://doi.org/10.1023/B:APPA.0000032927.78170.c1
- Klingenberg, C.P. (2010) There’s something afoot in the evolution of ontogenies. BMC Evolutionary Biology, 10, 221. https://doi.org/10.1186/1471-2148-10-221
- Klingenberg, C.P. (2011) Morpho J: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 11 (2), 353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
- Klingenberg, C.P. (2016) Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution, 226 (3), 113–137. https://doi.org/10.1007/s00427-016-0539-2
- Klingenberg, C.P. & Marugan-Lobon, J. (2013) Evolution covariation in geometric morphometric data: analyzing integration, modularity and allometry in a phylogenetic context. Systematic Biology, 62, 591–610. https://doi.org/10.1093/sysbio/syt025
- Klingenberg, C.P. & Gidaszewski, N.A. (2010) Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Systematic Biology, 59 (3), 245–261. https://doi.org/10.1093/sysbio/syp106
- Klingenberg, C.P. & McIntyre, G.S. (1998) Geometric morphometrics of developmental instability: analyzing the patterns of fluctuating asymmetry with Procrustes methods. Evolution, 52 (5), 1363–1375. https://doi.org/10.1111/j.1558-5646.1998.tb02018.x
- Knowlton, N. (1993) Sibling species in the sea. Annual Review of Ecology and Systematics, 24, 189–216. https://doi.org/10.1146/annurev.es.24.110193.001201
- Kornilios, P., Kumlutas, Y., Lymberakis, P. & Ligaz, C. (2018) Cryptic diversity and molecular systematics of the Aegean Ophiomorus skinks (Reptilia: Squamata), with the description of a new species. Journal of Zoological Systematics and Evolutionary Research, 56 (3), 364–381. https://doi.org/10.1111/jzs.12205
- Krantz, G. W. & Walter, D. E. (2009) A manual of acarology. 3rd Edition. Texas Tech University Press, Lubbock, Texas, 807 pp.
- Kristoffersen, J.B. & Magoulas, A. (2008) Investigating anchovy (Engraulis encrasicolus L.) population structure in the Mediterranean Sea using multiple methods. Fisheries Research, 91 (2–3), 187–195.
- https://doi.org/10.1016/j.fishres.2007.11.024
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35 (6), 1547–1549. https://doi.org/10.1093/molbev/msy096
- Laurin, M. (2004) The evolution of body size, Copes rule and the origin of amniotes. Systematic Biology, 53 (4), 594–622. https://doi.org/10.1080/10635150490445706
- Leache, A.D. & Mulcahy, D.G. (2007) Phylogeny, divergence times and species limits of spiny lizards (Scleroporus magister species group) in western North American deserts and Baja California. Molecular Ecology, 16 (24), 5216–5233.
- https://doi.org/10.1111/j.1365-294X.2007.03556.x
- Leslie, J.K. & Morre, J.E. (1986) Changes in lengths of fixed and preserved young freshwater fishes. Canadian Journal of Fisheries and Aquatic Sciences, 43 (5), 1079–1081. https://doi.org/10.1139/f86-136
- Lewandowski, M., Skoracka, A., Szydlo, W., Kozak, M., Druciarek, T. & Griffiths, D.A. (2014) Genetic and morphological diversity of Trisetacus species (Eriophyoidea: Phytoptidae) associated with coniferous trees in Poland: phylogeny, barcoding, host and habitat specialization. Experimental and Applied Acarology, 63, 497–520. https://doi.org/10.1007/s10493-014-9805-z
- Li, H.S., Xue, X.F. & Hong, X.Y. (2014) Cryptic diversity in host-associated populations of Tetra pinnatifidae (Acari: Eriophyoidea): What to morphometric, mitochondrial and nuclear data reveal and conceal?. Bulletin of Entomological Research, 104 (2), 221–232. https://doi.org/10.1017/S0007485313000746
- Liu, G.H., Chen, F., Chen, Y.Z., Song, H.Q., Lin, R.Q., Zhou, D.H. & Zhu, X.Q. (2013) Complete mitochondrial genome sequence data provides genetic evidence that the brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) represents a species complex. International Journal of Biological Sciences, 9 (4), 361–369. https://doi.org/10.7150.ijbs.6081
- Loiseau, C., Harrigan, R.J., Robert, A., Bowie, R.C.K., Thomassen, H.A., Smith, T.B. & Sehgal, R.N.M. (2012) Host and habitat specialization of avian malaria in Africa. Molecular Ecology, 21 (2), 431–441. https://doi.org/10.1111/j.1365-294X.2011.05341.x
- Martinez, P., Berbel-Filho, W.M. & Jacobina, U.P. (2012) Is formalin fixation and ethanol preservation able to influence in geometric morphometric analysis? Fishes as a case study. Zoomorphology, 132, 87–93. https://doi.org/10.1007/s00435-012-0176-x
- Matsuda, T., Morishita, M., Hinomoto, N. & Gotoh, T. (2014) Phylogenetic analysis of the spider mite sub-family Tetranychinae (Acari: tetranychidae) based on the mitochondrial COI gene and the 18S and the 5’ end of the 28S rRNA genes indicates that several general are polyphyletic. Plos One, 9 (10), e108672.
- https://doi.org/10.1371/journal.pone.0108672
- Miller, A.D., Skoracka, A., de Mendonca, R.S., Szydlo, W., Schultz, M.B., Smith, C.M., Truol, G. & Hoffmann, A.A. (2013) Phylogenetic analyses reveal extensive cryptic speciation and host specialization in an economically important mite taxon. Molecular Phylogenetics and Evolution, 66 (3), 928–940. https://doi.org/10.1016/j.ympev.2012.11.02
- Moore, W. (1995) Inferring phylogenies from mtDNA variation: mitochondrial-gene trees versus nuclear-gene trees. Evolution, 49 (4), 718–726. https://doi.org/10.1111/j.1558-5646.1995.tb02308.x
- Nadeau, J.L., Curtis, J.M.R. & Lourie, S.A. (2009) Preservation causes shrinkage in seahorses: implications for biological studies and for managing sustainable trade with minimum size limits. Aquatic Conservation Marine and Freshwater Ecosystems, 19 (4), 428–438. https://doi.org/10.1002/aqc.1002
- Navajas, M. & Boursot, P. (2003) Nuclear ribosomal DNA monophyly versus mitochondrial DNA polyphyly in two closely related mite species: the influence of life history and molecular drive. Proceeding of the Royal Society of London B, 270 (Supplement 1), 124–127. https://doi.org/10.1098/rsbl.2003.0034
- Navia, D., Moraes, G.J. & Querino, R.B. (2006) Geographic variation in the coconut mites, Acaeria guerreronis Keifer (Acari: Eriophyidae): a geometric morphometric analysis. International Journal of Acarology, 32 (3), 301–314. https://doi.org/10.1080/01647950608684473
- Oku, K. (2013) Sexual selection and mating behavior in spider mites of the genus Tetranychus (Acari: Tetranychidae). Applied Entomology and Zoology, 49, 1–9. https://doi.org/10.1007/s13355-013-0238-7
- Palmer, A.R. (1994) Fluctuating asymmetry analysis: a primer. In: Markow, T.A. (Eds.). Developmental Instability: its origins and evolutionary implications. Springer, Dordrecht, pp. 335–364. https://doi.org/10.1007/978-94-011-0830-0_26
- Pelabon, C., Firmat, C., Bolstad, G.H., Voje, K.L., Houle, D., Cassara, J., Le Rouzic, A. & Hansen, T.F. (2014) Evolution of morphological allometry. Annals of the New York Academy of Sciences, 1320 (1), 58–75. https://doi.org/10.1111/nyas.12470
- Polak, M. (2003) Developmental instability: causes and consequences. Oxford University Press, Oxford, 459 pp.
- Pozzebon, A., Duso, C. & Pavanetto, E. (2002) Side effects of some fungicide on phytoseiid mites (Acari, Phytoseiidae) in north-Italian vineyards. Journal of Pest Science, 75 (5), 132–136. https://doi.org/10.1046/j.1472-8206.2002.02037.x
- Premoli, A.C. (1996) Leaf architecture of south American Nothofagus (Nothofagaceae) using traditional and new methods in morphometrics. Botanical Journal of Linnean Society, 121 (1), 25–40.
- https://doi.org/10.1111/j.1095-8339.1996.tb00743.x
- Pretorius, E. & Clarke, F.C. (2000) Geometric morphometric analyses of the male and female body shape of Hyalomma truncatum and H. marginatum rufipes (Acari: Ixodidae). International Journal of Acarology, 26 (3), 229–238. https://doi.org/10.1080/01647950008684193
- Pringle, A., Baker, D.M., Platt, J.L., Wares, J.P., Latge, J.P. & Taylor, J.W. (2005) Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution, 59 (9), 1886–1899.
- Radenkovic, S., Zoric, L.S., Djan, M., Vidakovic, D.O., Acanski, J., Stahls, G., Velickovic, N., Markov, Z., Petanidou, T., Tubic, N.K. & Vujic, A. (2018) Cryptic speciation in the Merodon luteomaculatus complex (Diptera: Syrphidae) from the eastern Mediterranean. Journal of Zoological Systematics and Evolutionary Research, 56 (2), 170–191. https://doi.org/10.1111/jzs.12193
- Roda, A., Nyrop, J., English-Leop, G. & Dicke, M. (2001) Leaf pubescence and spotted spider mite webbing influence phytoseiid behaviour and population density. Oecologia, 129, 551–560. https://doi.org/10.1007/s004420100762
- Rohlf, F.J. (1999) Shape statistics: procrustes superimpositions and tangent spaces. Journal of Classification, 16 (2), 197–223. https://doi.org/10.1007/s003579900054
- Rohlf, F.J. (2015) The tps series of software. Hystrix The Italian Journal of Mammalogy, 26 (1), 9–12. https://doi.org/10.4404/hystrix-26.1-11264
- Rohlf, F.J. & Marcus, L.F. (1993) A revolution in morphometrics. Trends in Ecology and Evolution, 8 (4), 129–132. https://doi.org/10.1016/0169-5347(93)90024-J
- Rohlf, F.J. & Slice, D. (1990) Extensions of Procrustes method for the optimal superimposition of landmarks. Systematic Biology, 39 (1), 40–59. https://doi.org/10.2307/2992207
- Rohlf, F.J. (2003) tpsSmall: calculation of shape variation. Version 1.34. Department of Ecology and Evolution, State University of New York, Stony Brook, New York. Available from: https://life2.bio.sunysb.edu/ee/rohlf/software.html (accessed 12 April 2021)
- Roy, L., Dowling, A.P.G., Chauve, C.M. & Buronfosse, T. (2009) Delimiting species boundaries within Dermanyssus Duges, 1834 (Acari: Dermanyssidae) using a total evidence approach. Molecular Phylogenetics and Evolution, 50 (3), 446–470. https://doi.org/10.1016/j.ympev.2008.11.012
- Sadeghi, E., Shoushtari, R.V. & Madani, H. (2016) The influence of Tetranychus urticae Koch (Acari: Tetranychidae) life table and reproductive parameters by applying Si on Bean at library condition. Advances in Entomology, 4 (5), 260–267. https://doi.org/10.4236/ae.2016.45027
- Saez, A.G. & Lozano, E. (2005) Body doubles. Nature, 433 (111). [published online] https://doi.org/10.1038/433111a
- Saito, Y. (2009) Spider mites as study objects for evolutionary biology. Trends in Acarology: Proceedings of the 12th International Congress, 2009, 287–293. https://doi.org/10.1007/978-90-481-9837-5_46
- Saito, Y., Mori, K. & Chittenden, A.R. (1999) Body characters reflecting the body size of spider mites in flattened specimens (Acari, Tetranchychidae). Applied Entomology and Zoology, 34 (3), 383–386. https://doi.org/10.1303/aez.34.383
- Sakagami, T., Saito, Y., Kongchuensin, M. & Sahara, K. (2009) Molecular phylogeny of Stigmaeopsis, with special reference to speciation through host plant shift. Annals of the Entomological Society of America, 102 (3), 360–366.
- https://doi.org/10.1603/008.102.0303
- Samadi, S. & Barberousse, A. (2009) Species: towards new, well-grounded practices. Biological Journal of Linnean Society, 97 (1), 217–222.
- https://doi.org/10.1111/j.1095-8312.2009.01191.x
- Sanger, F. & Coulson, A.R. (1975) A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. Journal of Molecular Biology, 94 (3), 441–448. https://doi.org/10.1016/0022-2836(75)90213-2
- Sato, Y. & Saito, Y. (2008) Evolutionary view of waste management behavior using chemical cues in social spider mites. Journal of Ethology, 26, 267–272. https://doi.org/10.1007/s10164-007-0069-5
- Schaffer, S., Pfingstl, T., Kobmuller, S., Winkler, K. A., Sturmbauer, C. & Krisper, G. (2010) Phylogenetic analysis of European Scutovertex mites (Acari, Oribatida, Scutoverticidae) reveals paraphyly and cryptic diversity: a molecular genetic and morphological approach. Molecular Phylogenetics and Evolution, 55 (2), 677–688. https://doi.org/10.1016/j.ympev.2009.11.025
- Schausberger, P. (1997) Inter-and intraspecific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri, and Kampimodormus aberrans (Acarina, Phytoseiidae). Experimental and Applied Acarology, 21 (3), 131–15. https://doi.org/10.1023/A:1018478418010
- Schausberger, P. & Croft, B.A. (2000) Cannibalism and intraguild predation among phytoseiid mites: are aggressiveness and prey preference related to diet specialization? Experimental and Applied Acarology, 24, 709–725. https://doi.org/10.1023/A.1010747208519
- Schluter, D. (2001) Ecology and the origin of species. Trends in Ecology and Evolution, 16 (7), 372–380. https://doi.org/10.1016/S0169-5347(01)02198-X
- Schmidt, E.J., Parsons, T.E., Jamniczky, H.A. Gitelman, J., Trpkov, C., Boughner, J.C., Logan, C.C., Sensen, C.W. & Hallgrimsson, B. (2010) Micro-computed tomography-based phenotypic approaches in embryology: procedural artifacts on assessments of embryonic craniofacial growth and development. BMC Developmental Biology, 10, 18. https://doi.org/10.1186/1471-213X-10-18
- Seeman, O.D. & Beard, J.J. (2011) Identification of exotic pest and Australian native and naturalized species of Tetranychus (Acari: Tetranychidae). Zootaxa, 2961 (1), 1–72. https://doi.org/10.11646/zootaxa.2961.1.1
- Shaw, K.L. (2002) Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: what mtDNA reveals and conceals about modes of speciation in Hawaiian crickets. PNAS, 99 (25), 16122–16127. https://doi.org/10.1073/pnas.242585899
- Shields, P.A. & Carlson, S.R. (1996) Effects of formalin and alcohol preservation on length and weights of juvenile sockeye salmon. Alaska Fishery Research Bulletin, 3 (2), 81-93.
- Skoracka, A. (2008) Reproductive barriers between populations of the cereal rust mite Abacarus hystrix confirm their host specialization. Evolutionary Ecology, 22, 607–616. https://doi.org/10.1007/s10682-007-9185-5
- Skoracka, A. & Dabert, M. (2010) The cereal rust mite Abacarus hystrix (Acari: Eriophyoidea) is a complex of species: evidence from mitochondrial and nuclear sequences. Bulletin of Entomological Research, 100 (3), 263–272. https://doi.org/10.1017/S0007485309990216
- Skoracka, A., Kuczynski, L. & Magowski, W. (2002) Morphological variation in different host populations of Abacarus hystrix (Nalepa, 1896) (Acari: Prostigmata: Eriophyoidea). Experimental and Applied Acarology, 26, 187–193. https://doi.org/10.1023/A:1021144729837
- Skoracka, A., Kuczynski, L., Rector, B. & Amrine, J.W. Jr. (2014) Wheat curl mite and dry bulb mite: untangling a taxonomic conundrum through a multidisciplinary approach. Biological Journal of Linnean Society, 111 (2), 421–436.
- https://doi.org/10.1111/bij.12213
- Skoracka, A., Kuczynski, L., de Santos, M.R., Dabert, M., Szydlo, W., Knihinick, D., Truol, G. & Navia, D. (2012) Cryptic species within the wheat curl mite Aceria tosichella (Keifer) (Acar: Eriophyoidea), revealed by mitochondrial, nuclear and morphometric data. Invertebrate Systematics, 26 (4), 417–433. https://doi.org/10.1071/IS11037
- Skoracka, A., Magalhaes, S., Rector, B.G. & Kuczynski, L. (2015) Cryptic speciation in the Acari: a function of species lifestyles or our ability to separate species? Experimental and Applied Acarology, 67, 165–182. https://doi.org/10.1007/s10493-015-9954-8
- Steinauer, M.L., Nickol, B.B. & Orti, G. (2007) Cryptic speciation and patterns of phenotypic variation of a highly variable acanthocephalan parasite. Molecular Ecology, 16 (199), 4097–4109. https://doi.org/10.1111/j.1365-294X.2007.03462.x
- Stireman, J.O. III., Devlin, H., Carr, T.G. & Abbot, P. (2010) Evolutionary diversification of the gall midge genus Asteromyia (Cecidomyiidae) in a multitrophic ecological context. Molecular Phylogenetics and Evolution, 54 (1), 194–210. https://doi.org/10.1016/j.ympev.2009.09.010
- Stireman, J.O. III., Nason, J.D. & Heard, S.B. (2005) Host associated genetic differentiation in phytophagous insects: general phenomenon or isolated exceptions? Evidence from a goldenrod-insect community. Evolution, 59 (12), 2573-2587. https://doi.org/10.1554/05-222.1
- Tajima, F. (1993) Simple methods for testing molecular clock hypothesis. Genetics, 135 (2), 599–607. https://doi.org/10.1093/genetics/135.2.599
- Tamar, K., Mitsi, P. & Carranza, S. (2018) Cryptic diversity related in the leaf-toed gecko Asacus montanus (Squamata, Phyllodactylidae) from the Hajar Mountains of Arabia. Journal of Zoological Systematics and Evolutionary Research, 52 (2), 1–14. https://doi.org/10.1111/jzs.12258
- Tamura, K., Battistuzzi, F. U., Billing-Ross, P., Murillo, O., Filipski, A. & Kumar, S. (2012) Estimating divergence times in large molecular phylogenies. PNAS, 109 (47), 19333–19338. https://doi.org/10.1073/pnas.1213199109
- Tamura, K. & Nei, M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10 (3), 512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
- Thornhill, R. & Gangestad, S.W. (2008) The evolutionary biology of human female sexuality. Oxford University Press, Oxford, 424 pp.
- Treasurer, J.W. (1992) Length and weight changes in O + perch, Perca fluviatilis L. following fixation in formalin. Journal of Fish Biology, 41 (6), 1033–1036. https://doi.org/10.1111/j.1095-8649.1992.tb02730.x
- Tsang, L.M., Chan, B.K.K., Shih, F.L., Chu, K.H. & Allen, C.C. (2009) Host-associated speciation in the coral barnacle Wanella milleporae (Cirripedia: Pyrgomatidae) inhabiting the Millepora coral. Molecular Ecology, 18 (7), 1463–1475. https://doi.org/10.1111/j.1365-294X.2009.04090.x
- Tuovinen, T. (1994) Influence of surrounding trees and bushes on the phytoseiid mite fauna on apple orchard tress in Finland. Agriculture Ecosystems and Environment, 50 (1), 39–47. https://doi.org/10.1016/0167-8809(94)90123-6
- Valen, L.V. (1962) A study of fluctuating asymmetry. Evolution, 16 (2), 125–142. https://doi.org/10.1111/j.1558-5646.1962.tb03206.x
- Vasconcelos, G.J.N., Silva, F.R. da., Gondim, M.G.C. Jr., Barros, R. & Oliveira, J.V. (2004) Effects of different temperatures on the development and reproduction of Tetranychus abacae Baker and Pritchard (Acari: Tetranychidae) on Musa sp. cv. Prata. Neotropical Entomology, 33 (2), 149–154.
- Vidovic, B., Jojic, V., Maric, I., Marinkovic, S., Hansen, R. & Petanovic, R. (2014) Geometric morphometric study of geographic and host-related variability in Aceria spp. (Acari: Eriophyoidea) inhabiting Cirsium spp. (Asteraceae). Experimental and Applied Acarology, 64, 321–335. https://doi.org/10.1007/s10493-014-9829-4
- Villemant, C., Simbolotti, G. & Kenis, M. (2007) Discrimination of Eubazus (Hymenoptera, Braconidae) sibling species using geometric morphometrics analysis of wing venation. Systematic Entomology, 32 (4), 625–634. https://doi.org/10.1111/j.1365-3113.2007.00389.x
- Walter, D.E. & Proctor, H.C. (2013) Mites-ecology, evolution and behaviour: life at a microscale. 2nd Edition. Springer, Dordrecht, XIV + 494 pp.
- Wermelinger, B., Oertli, J. J. & Baumgartner, J. (1991) Environmental factors affecting the life-tables of Tetranychus urticae (Acari: Tetranychidae). III. Host-plat nutrition. Experimental and Applied Acarology, 12, 259–274. https://doi.org/10.1007/BF01193472
- Zemek, R. (1993) Characteristic of development and reproduction in Typhlodromus pyri on Tetranychus urticae and Cecidophyopsis ribis. I. Overwintered females. Experimental and Applied Acarology, 17, 405–521. https://doi.org/10.1007/BF00120499
- Zhang, Z-Q. (2011) Animal biodiversity: an introduction to higher-level classification and taxonomic richness. Zootaxa, 3148 (1), 7–12. https://doi.org/10.11646/zootaxa.3148.1.3
- Zikic, V., Stankovic, S.S., Petrovic, A., Milosevic, M., Tomanovic, Z., Klingenberg, C.P. & Ivanovic, A. (2017) Evolutionary relationship of wing venation and wing size and shape in Aphidiinae (Hymenoptera: Braconidae). Organisms and Diversity Evolution, 17, 607–617. https://doi.org/10.1007/s13127-017-0338-2