Abstract
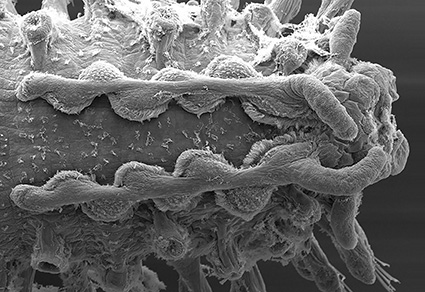
The phylogenetic relationships of Syllidae have been analyzed in several studies during the last decades, resulting in highly congruent topologies. Most of the subfamilies were found to be monophyletic, while other groups (Eusyllinae and several genera) have been reorganized attending their phylogenetic relationships. However, there are still several enigmatic genera, which could not be assigned to any of the established subgroups. These enigmatic genera usually show a combination of characters indicating relationships with several different groups, and some show morphological traits unique to Syllidae. One of the most intriguing genera, still unclassified within Syllidae is Clavisyllis Knox. Herein, we provide a complete description of a new species Clavisyllis tenjini n. sp. from Japan. We sequence the complete mitochondrial genome, compare with the available data from other syllids, and perform a phylogenetic analysis of three genes (18S, 16S, COI), traditionally used in previous studies. Clavisyllis shows a unique combination of characters within Syllidae, such as nuchal lappets and large ovoid dorsal cirri. The new species has additional anterior appendages that have not been found in any other syllid. Our results show the genus is a member of Eusyllinae, closely related to Pionosyllis Malmgren. The mitochondrial gene order agrees with the considered plesiomorphic gene order in Annelida, which is present in all members of Eusyllinae investigated so far. Clavisyllis reproduces by epigamy, the reproductive mode of members of Eusyllinae. The present study contributes to the systematics of Syllidae, a complex group with a large number of species and striking reproductive modes.
References
Aguado, M.T. & Glasby, C.J. (2015) Indo-Pacific Syllidae (Annelida, Phyllodocida) share an evolutionary history. Systematics and Biodiversity, 13, 369–385. https://doi.org/10.1080/14772000.2014.992379
Aguado, M.T., Glasby, C.J., Schroeder, P.C., Weigert, A. & Bleidorn, C. (2015a) The making of a branching annelid: An analysis of complete mitochondrial genome and ribosomal data of Ramisyllis multicaudata. Scientific Reports, 5, 1–13. https://doi.org/10.1038/srep12072
Aguado, M.T., Helm, C., Weidhase, M. & Bleidorn, C. (2015b) Description of a new syllid species as a model for evolutionary research of reproduction and regeneration in annelids. Organisms Diversity and Evolution, 15, 1–21. https://doi.org/10.1007/s13127-014-0183-5
Aguado, M.T., Nygren, A. & Siddall, M.E. (2007) Phylogeny of Syllidae (Polychaeta) based on combined molecular analysis of nuclear and mitochondrial genes. Cladistics, 23, 552–564. https://doi.org/10.1111/j.1096-0031.2007.00163.x
Aguado, M.T., Ponz-Segrelles, G., Glasby, C.J., Ribeiro, R.P., Nakamura, M., Oguchi, K., Omori, A., Kohtsuka, H., Fisher, C., Ise, Y., Jimi, N. & Miura, T. (2022) Ramisyllis kingghidorahi n. sp., a new branching annelid from Japan. Organisms Diversity & Evolution, 22, 377–405. https://doi.org/10.1007/s13127-021-00538-4
Aguado, M.T., Richter, S., Sontowski, R., Golombek, A., Struck, T.H. & Bleidorn, C. (2016) Syllidae mitochondrial gene order is unusually variable for Annelida. Gene, 594, 89–96. https://doi.org/10.1016/j.gene.2016.08.050
Aguado, M.T. & San Martín, G. (2008) Re-description of some enigmatic genera of Syllidae (Phyllodocida: Polychaeta). Journal of the Marine Biological Association of the United Kingdom, 88, 35–56. https://doi.org/10.1017/S002531540800026X
Aguado, M.T. & San Martín, G. (2009) Phylogeny of Syllidae (Polychaeta) based on morphological data. Zoologica Scripta, 38, 379–402. https://doi.org/10.1111/j.1463-6409.2008.00380.x
Aguado, M.T., Siddall, M.E. & San Martín, G. (2012) Systematics and evolution of syllids (Annelida, Syllidae). Cladistics, 28, 234–250. https://doi.org/https://doi.org/10.1111/j.1096-0031.2011.00377.x
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. (1990) Basic Local Alignment Search. Journal of Molecular Biology, 215, 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Bernt, M., Donath, A., Jühling, F., Externbrink, F., Florentz, C., Fritzsch, G., Pütz, J., Middendorf, M. & Stadler, P.F. (2013) MITOS: Improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution, 69, 313–319. https://doi.org/10.1016/j.ympev.2012.08.023
Cejp, B., Ravara, A. & Aguado, M.T. (2022) First mitochondrial genomes of Chrysopetalidae (Annelida) from shallow-water and deep-sea chemosynthetic environments. Gene, 815, 146–159. https://doi.org/10.1016/j.gene.2021.146159
Glasby, C.J. (1994) A new genus and species of polychaete, Bollandia antipathicola (Nereidoidea: Syllidae), from black coral. Proceedings of the Biological Society of Washington, 107, 615–621. https://doi.org/10.4039/Ent3213-1
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. (2014) Circlize implements and enhances circular visualization in R. Bioinformatics, 30, 2811–2812. https://doi.org/10.1093/bioinformatics/btu393
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. (2013) QUAST: Quality assessment tool for genome assemblies. Bioinformatics, 29, 1072–1075. https://doi.org/10.1093/bioinformatics/btt086
Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108
Katoh, K. & Standley, D.M. (2013) MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Molecular Biology and Evolution, 30, 772–780. https://doi.org/10.1093/molbev/mst010
Knox, G.A. (1957) Clavisyllis alternata , gen. et sp. nov., a new polychaete from New Zealand. Annals and Magazine of Natural History, 10, 493–496. https://doi.org/10.1080/00222935708655989
Kück, P. & Meusemann, K. (2010) FASconCAT: Convenient handling of data matrices. Molecular Phylogenetics and Evolution, 56, 1115–1118. https://doi.org/10.1016/j.ympev.2010.04.024
Lucas, Y., San Martín, G. & Sikorski, A. (2010) A new genus and species of Syllidae (Annelida: Polychaeta) from off the coast of Norway with unusual morphological characters and an uncertain systematic position. Proceedings of the Biological Society of Washington, 123, 251–257. https://doi.org/10.2988/09-11.1
Martin, D., Alos, C. & Sarda, R. (1990) Miscellania dentata gen. et sp.n. (Polychaeta: Syllidae) from the Spanish Mediterranean coast. Zoologica, 19, 169–172. https://doi.org/10.1111/j.1463-6409.1990.tb00251.x
Martin, D., Marin, I. & Britayev, T.A. (2008) Features of the first known association between Syllidae (Annelida, Polychaeta) and crustaceans. Organisms Diversity and Evolution, 8, 279–281. https://doi.org/10.1016/j.ode.2007.12.002
Meyer, M. & Kircher, M. (2010) Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harbor protocols, 2010, pdb.prot5448. https://doi.org/10.1101/pdb.prot5448
Nguyen, L.T., Schmidt, H.A., Von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300
Nygren, A. & Sundberg, P. (2003) Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Molecular Phylogenetics and Evolution, 29, 235–249. https://doi.org/10.1016/S1055-7903(03)00095-2
Pamungkas, J., Glasby, C.J., Read, G.B., Wilson, S.P. & Costello, M.J. (2019) Progress and perspectives in the discovery of polychaete worms (Annelida) of the world. Helgoland Marine Research, 73, 4. https://doi.org/10.1186/s10152-019-0524-z
Peng, Y., Leung, H.C.M., Yiu, S.M. & Chin, F.Y.L. (2012) IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics, 28, 1420–1428. https://doi.org/10.1093/bioinformatics/bts174
Perna, N.T. & Kocher, T.D. (1995) Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. Journal of Molecular Evolution, 41, 353–358. https://doi.org/10.1007/BF00186547
Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. (2020) Using SPAdes De Novo Assembler. Current Protocols in Bioinformatics, 70, 1–29. https://doi.org/10.1002/cpbi.102
Read, G.B. & Fauchald , K. (Ed.) (2022) World Polychaeta Database. Syllidae Grube, 1850. Accessed through: World Register of Marine Species. Available from: https://www.marinespecies.org/aphia.php?p=taxdetails&id=948 (accessed 14 January 2023)
Renaud, G., Kircher, M., Stenzel, U. & Kelso, J. (2013) freeIbis: an efficient basecaller with calibrated quality scores for Illumina sequencers. Bioinformatics, 29, 1208–1209. https://doi.org/10.1093/bioinformatics/btt117
Renaud, G., Stenzel, U. & Kelso, J. (2014) leeHom: adaptor trimming and merging for Illumina sequencing reads. Nucleic acids research, 42, e141. https://doi.org/10.1093/nar/gku699
Ribeiro, R.P., Bleidorn, C. & Aguado, M.T. (2018) Regeneration mechanisms in Syllidae (Annelida). Regeneration, 5, 26–42. https://doi.org/10.1002/reg2.98
Ribeiro, R.P., Ponz-Segrelles, G., Helm, C., Egger, B. & Aguado, M.T. (2020) A new species of Syllis Grube, 1850 including transcriptomic data and an updated phylogeny of Syllinae (Annelida: Syllidae). Marine Biodiversity 50, 31. https://doi.org/10.1007/s12526-020-01046-y
Rivolta, A., San Martín, G. & Sikorski, A. (2016) Additions to the description, reproduction and systematic position of the enigmatic species Acritagasyllis longichaetosus Lucas, San Martín & Sikorski, 2010 (Annelida: Phyllodocida: Syllidae). Journal of the Marine Biological Association of the United Kingdom, 96, 1709–1716. https://doi.org/10.1017/S0025315415002118
San Martín, G. (2002) A new genus and species of Syllidae (Polychaeta) from Australia dorsally brooding eggs by means of compound notochaetae, with comments on external brooding in the family. Proceedings of the Biological Society of Washington, 115, 333–340.
San Martín, G. (2005) Exogoninae (Polychaeta: Syllidae) from Australia with the description of a new genus and twenty-two new species. Records of the Australian Museum, 57, 39–152. https://doi.org/10.3853/j.0067-1975.57.2005.1438
San Martín, G. & Aguado, M.T. (2012) Contribution of Scanning Electron Microscope to the Study of Morphology, Biology, Reproduction, and Phylogeny of the Family Syllidae (Polychaeta). In: Scanning Electron Microscopy. Intech Open, London, pp. 129–146.
San Martín, G. & Aguado, M.T. (2022) Syllidae Grube, 1850. In: Purschke, G., Böggemann, M. & Westheide, W. (Eds.), Pleistoannelida, Errantia II. De Gruyter, Berlin, pp. 152–308.
San Martín, G., Aguado, M.T. & Murray, A. (2007) A new genus and species of Syllidae (Annelida: Polychaeta) from Australia with unusual morphological characters and uncertain systematic position. Proceedings of the Biological Society of Washington, 120, 39–48. https://doi.org/10.2988/09-11.1
San Martín, G., Ibarzábal, D., Jiménez, M. & López, E. (1997) Redescription of Haplosyllides floridana Augener, 1924 (Polychaeta: Syllidae: Syllinae), with notes on morphological variability and comments on the generic status. Bulletin of Marine Science, 60 (2), 364–370.
Watson, C. (2009) A New Species of Clavisyllis Knox, 1957 (Polychaeta: Syllidae): A Genus with the Unusual Distribution of New Zealand and the Great Barrier Reef, Northern Queensland, Australia. The Beagle: Records of the Museums and Art Galleries of the Northern Territory, 25, 77–84.
Weigert, A., Golombek, A., Gerth, M., Schwarz, F., Struck, T.H. & Bleidorn, C. (2016) Evolution of mitochondrial gene order in Annelida. Molecular Phylogenetics and Evolution, 94, 196–206. https://doi.org/10.1016/j.ympev.2015.08.008
Xia, X. (2013) DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Molecular Biology and Evolution, 30, 1720–1728. https://doi.org/10.1093/molbev/mst064
Zhang, Z., Schwartz, S., Wagner, L. & Miller, W. (2000) A greedy algorithm for aligning DNA sequences. Journal of Computational Biology, 7, 203–214. https://doi.org/10.1089/10665270050081478