Abstract
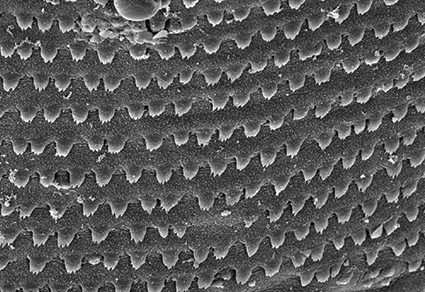
Species of the digenean genus Enenterum Linton, 1910 (Lepocreadioidea: Enenteridae) are characterised primarily by their elaborate oral suckers, which are divided into varying numbers of anteriorly directed lobes, and their host-restriction to herbivorous marine fishes of the family Kyphosidae. We describe Enenterum petrae n. sp. from the brassy chub Kyphosus vaigiensis (Quoy & Gaimard) collected off Lizard Island, Great Barrier Reef, Queensland, Australia. Enenterum petrae n. sp. is readily differentiated from congeners by its unique oral sucker morphology, in having a minute pharynx, and the combination of a genital cap and accessory sucker. We also provide the first record of Enenterum kyphosi Yamaguti, 1970 from Australia based on material obtained from the blue sea chub Kyphosus cinerascens (Forsskål) collected off Lizard Island and North Stradbroke Island, Queensland. Morphologically, our specimens of E. kyphosi agree closely with descriptions of this species from Hawaii and South Africa, and despite lack of molecular data from outside of Australian waters, we consider all three reports to represent a single, widespread species. The first ITS2 and COI mtDNA gene sequences for species of Enenterum are provided and molecular phylogenetic analyses of 28S rDNA gene sequences place these species in a strongly-supported clade with the type-species of the genus, Enenterum aureum Linton, 1910. The oral suckers of both E. kyphosi and E. petrae n. sp. can be interpreted as having varying numbers of lobes depending on the particular specimen and how the division between lobes is defined. Scanning electron microscopical images improves understanding of the morphology of the enenterid oral sucker, and permits speculation regarding the evolutionary history leading to its specialisation in this lineage.
References
Andres, M.J., Pulis, E.E., Curran, S.S. & Overstreet, R.M. (2018) On the systematics of some marine haploporids (Trematoda) with the description of a new species of Megasolena Linton, 1910. Parasitology International, 67, 805–815. https://doi.org/10.1016/j.parint.2018.08.002
DOI: https://doi.org/10.1016/j.parint.2018.08.002Bray, R.A. (1978) Two new species of Enenterum Linton, 1910 (Digenea) in the marine fish Neoscorpis lithophilus (Kyphosidae) from the south-western Indian Ocean. Journal of Helminthology, 52, 131–139. https://doi.org/10.1017/S0022149X00005253
DOI: https://doi.org/10.1017/S0022149X00005253Bray, R.A. (1986) Some helminth parasites of marine fishes of South Africa: families Enenteridae, Opistholebetidae and Pleorchiidae (Digenea). Journal of Natural History, 20, 471–488. https://doi.org/10.1080/00222938600770371
DOI: https://doi.org/10.1080/00222938600770371Bray, R.A. (2005) Family Enenteridae Yamaguti, 1958. In: Jones, A., Bray, R.A. & Gibson, D.I. (Eds.), Keys to the Trematoda. Vol. 2. CABI Publishing and the Natural History Museum, Wallingford, pp. 657–661.
DOI: https://doi.org/10.1079/9780851995878.0657Bray, R.A. & Cribb, T.H. (2001) A review of the family Enenteridae Yamaguti, 1958 (Digenea), with descriptions of species from Australian waters, including Koseiria huxleyi n. sp. Systematic Parasitology, 48, 1–29. https://doi.org/10.1023/A:1026533510387
DOI: https://doi.org/10.1023/A:1026533510387Bray, R.A. & Cribb, T.H. (2002) Further observations on the Enenteridae Yamaguti, 1958 (Digenea, Lepocreadioidea) of the Indo-West Pacific region, including a new species from Western Australia. Acta Parasitologica, 47, 208–223.
Bray, R.A., Cribb, T.H. & Cutmore, S.C. (2018a) Lepocreadiidae Odhner, 1905 and Aephnidiogenidae Yamaguti, 1934 (Digenea: Lepocreadioidea) of fishes from Moreton Bay, Queensland, Australia, with the erection of a new family and genus. Systematic Parasitology, 95, 479–498. https://doi.org/10.1007/s11230-018-9803-3
DOI: https://doi.org/10.1007/s11230-018-9803-3Bray, R.A., Cribb, T.H., Waeschenbach, A. & Littlewood, D.T.J. (2014) Molecular evidence that the genus Cadenatella Dollfus, 1946 (Digenea: Plagiorchiida) belongs in the superfamily Haploporoidea Nicoll, 1914. Systematic Parasitology, 89, 15–21. https://doi.org/10.1007/s11230-014-9504-5
DOI: https://doi.org/10.1007/s11230-014-9504-5Bray, R.A., Cutmore, S.C. & Cribb, T.H. (2018b) Lepotrema Ozaki, 1932 (Lepocreadiidae: Digenea) from Indo-Pacific fishes, with the description of eight new species, characterised by morphometric and molecular features. Systematic Parasitology, 95, 693–741. https://doi.org/10.1007/s11230-018-9821-1
DOI: https://doi.org/10.1007/s11230-018-9821-1Bray, R.A., Cutmore, S.C. & Cribb, T.H. (2022) A paradigm for the recognition of cryptic trematode species in tropical Indo-west Pacific fishes: the problematic genus Preptetos (Trematoda: Lepocreadiidae). International Journal for Parasitology, 52, 169–203. https://doi.org/10.1016/j.ijpara.2021.08.004
DOI: https://doi.org/10.1016/j.ijpara.2021.08.004Bray, R.A., Waeschenbach, A., Cribb, T.H., Weedall, G.D., Dyal, P. & Littlewood, D.T.J. (2009) The phylogeny of the Lepocreadioidea (Platyhelminthes, Digenea) inferred from nuclear and mitochondrial genes: implications for their systematics and evolution. Acta Parasitologica, 54, 310–329. https://doi.org/10.2478/s11686-009-0045-z
DOI: https://doi.org/10.2478/s11686-009-0045-zBrooks, D.R., Pérez-Ponce de León, G., León-Régàgnon, V. (2000) Phylogenetic analysis of the Enenterinae (Digenea, Lepocreadiidae) and discussion of the evolution of the digenean fauna of kyphosid fishes. Zoologica Scripta, 29, 237–246. https://doi.org/10.1046/j.1463-6409.2000.00042.x
DOI: https://doi.org/10.1046/j.1463-6409.2000.00042.xCribb, T.H. & Bray, R.A. (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7. https://doi.org/10.1007/s11230-010-9229-z
DOI: https://doi.org/10.1007/s11230-010-9229-zCribb, T.H., Bray, R.A., Diaz, P.E., Huston, D.C., Kudlai, O., Martin, S.B., Yong, R.Q.-Y. & Cutmore, S.C. (2016) Trematodes of fishes of the Indo-west Pacific: told and untold richness. Systematic Parasitology, 93, 237–247. https://doi.org/10.1007/s11230-016-9625-0
DOI: https://doi.org/10.1007/s11230-016-9625-0Dollfus, R.P. (1946) Sur trois espèces de distomes, dont une a 17 ventouses (Enenterum (Jeancadenatia) brumpti n. sp.) parasites du poisson marin Kyphosus sectatrix (L.). Annales de Parasitologie Humaine et Comparée, 21, 119–128. https://doi.org/10.1051/parasite/1946213119
DOI: https://doi.org/10.1051/parasite/1946213119Dyer, W.G., Williams, E.H. & Bunkley-Williams, L. (1992) Homalometron dowgialloi sp. n. (Homalometridae) from Haemulon flavolineatum and additional records of digenetic trematodes of marine fishes in the West Indies. Journal of the Helminthological Society of Washington, 59, 182–189.
Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340
DOI: https://doi.org/10.1093/nar/gkh340Fischthal, J.H. & Thomas, J.D. (1972) Digenetic trematodes of marine fishes from Senegal. Bulletin de l'Institut Fondamental d'Afrique Noire, 34A, 292–322.
Hall, K.A., Chambers, C. & Jones, M.K. (1999) A new genus of the Gyliauchenidae Goto et Matsudaira, 1918 (Digenea) from Naso tuberosus (Percomorpha, Acanthuridae) on the Great Barrier Reef, Queensland, Australia. Acta Parasitologica, 44, 229–232.
Hafeezullah, M. (1980) Two digenetic trematodes of a marine fish, Kyphosus cinerascens (Forskal), from the Gulf of Mannar with note on the systematic positions of the genera Enenterum Linton, 1910, Cadenatella Dollfus, 1946 and Jeancadenatia Dollfus, 1946. Bulletin of the Zoological Survey of India, 2, 145–151.
Huston, D.C., Cribb, T.H. & Welicky, R.L. (2021a) Stable isotope signatures of an acanthocephalan and trematode from the herbivorous marine fish Kyphosus bigibbus (Perciformes: Kyphosidae). Journal of Parasitology, 107, 726–730. https://doi.org/10.1645/21-29
DOI: https://doi.org/10.1645/21-29Huston, D.C., Cutmore, S.C. & Cribb, T.H. (2016) The life-cycle of Gorgocephalus yaaji Bray & Cribb, 2005 (Digenea: Gorgocephalidae) with a review of the first intermediate hosts for the superfamily Lepocreadioidea Odhner, 1905. Systematic Parasitology, 93, 653–665. https://doi.org/10.1007/s11230-016-9655-7
DOI: https://doi.org/10.1007/s11230-016-9655-7Huston, D.C., Cutmore, S.C. & Cribb, T.H. (2019a) An identity crisis in the Indo-Pacific: molecular exploration of the genus Koseiria (Digenea: Enenteridae). International Journal for Parasitology, 49, 945–961. https://doi.org/10.1016/j.ijpara.2019.07.001
DOI: https://doi.org/10.1016/j.ijpara.2019.07.001Huston, D.C., Cutmore, S.C. & Cribb, T.H. (2019b) A new genus and species of the trematode family Gyliauchenidae Fukui, 1929 from an unexpected, but plausible, host, Kyphosus cornelii (Perciformes: Kyphosidae). Parasitology, 146, 937–946. https://doi.org/10.1017/S0031182019000118
DOI: https://doi.org/10.1017/S0031182019000118Huston, D.C., Cutmore, S.C., Miller, T.L., Sasal, P., Smit, N.J. & Cribb, T.H. (2021b) Gorgocephalidae (Digenea: Lepocreadioidea) in the Indo-West Pacific: new species, life-cycle data and perspectives on species delineation over geographic range. Zoological Journal of the Linnean Society, 193, 1416–1455. https://doi.org/10.1093/zoolinnean/zlab002
DOI: https://doi.org/10.1093/zoolinnean/zlab002Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., Von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589. https://doi.org/10.1038/nmeth.4285
DOI: https://doi.org/10.1038/nmeth.4285Knudsen, S.W., Choat, J.H. & Clements, K.D. (2019) The herbivorous fish family Kyphosidae (Teleostei: Perciformes) represents a recent radiation from higher latitudes. Journal of Biogeography, 46, 2067–2080. https://doi.org/10.1111/jbi.13634
DOI: https://doi.org/10.1111/jbi.13634Knudsen, S.W. & Clements, K.D. (2013a) Kyphosus gladius, a new species of sea chub from Western Australia (Teleostei: Kyphosidae), with comments on Segutilum klunzingeri Whitley. Zootaxa, 3599 (1), 1–18. https://doi.org/10.11646/zootaxa.3599.1.1
DOI: https://doi.org/10.11646/zootaxa.3599.1.1Knudsen, S.W. & Clements, K.D. (2013b) Revision of the fish family Kyphosidae (Teleostei: Perciformes). Zootaxa, 3751 (1), 1–101. https://doi.org/10.11646/zootaxa.3751.1.1
DOI: https://doi.org/10.11646/zootaxa.3751.1.1Knudsen, S.W. & Clements, K.D. (2016) World-wide species distributions in the family Kyphosidae (Teleostei: Perciformes). Molecular Phylogenetics and Evolution, 101, 252–266. https://doi.org/10.1016/j.ympev.2016.04.037
DOI: https://doi.org/10.1016/j.ympev.2016.04.037Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
DOI: https://doi.org/10.1093/molbev/msy096Linton, E. (1910) Helminth fauna of the Dry Tortugas. II. Trematodes. Papers from the Tortugas Laboratory of the Carnegie Institute of Washington, 4, 11–98.
Lo, C.M., Morgan, J.A.T., Galzin, R. & Cribb, T.H. (2001) Identical digeneans in coral reef fishes from French Polynesia and the Great Barrier Reef (Australia) demonstrated by morphology and molecules. International Journal for Parasitology, 31, 1573–1578. https://doi.org/10.1016/S0020-7519(01)00306-X
DOI: https://doi.org/10.1016/S0020-7519(01)00306-XMachida, M. (1993) Trematodes from kyphosid fishes in Japanese and adjacent waters. Bulletin of the National Science Museum, Tokyo Series A Zoology, 19, 27–36.
Manter, H.W. (1947) The digenetic trematodes of marine fishes of Tortugas, Florida. American Midland Naturalist, 38, 257–416. https://doi.org/10.2307/2421571
DOI: https://doi.org/10.2307/2421571Manter, H.W. (1954) Some digenetic trematodes from fishes of New Zealand. Transactions of the Royal Society of New Zealand, 82, 475–568.
Martin, S.B., Huston, D.C., Cutmore, S.C. & Cribb, T.H. (2019) A new classification for deep-sea opecoelid trematodes based on the phylogenetic position of some unusual taxa from shallow-water, herbivorous fishes off south-west Australia. Zoological Journal of the Linnean Society, 186, 385–413. https://doi.org/10.1093/zoolinnean/zly081
DOI: https://doi.org/10.1093/zoolinnean/zly081Martin, S.B., Sasal, P., Cutmore, S.C., Ward, S., Aeby, G.S. & Cribb, T.H. (2018) Intermediate host switches drive diversification among the largest trematode family: evidence from the Polypipapiliotrematinae n. subf. (Opecoelidae), parasites transmitted to butterflyfishes via predation of coral polyps. International Journal for Parasitology, 48, 1107–1126. https://doi.org/10.1016/j.ijpara.2018.09.003
DOI: https://doi.org/10.1016/j.ijpara.2018.09.003McNamara, M.K.A., Miller, T.L. & Cribb, T.H. (2014) Evidence for extensive cryptic speciation in trematodes of butterflyfishes (Chaetodontidae) of the tropical Indo-West Pacific. International Journal for Parasitology, 44, 37–48. https://doi.org/10.1016/j.ijpara.2013.09.005
DOI: https://doi.org/10.1016/j.ijpara.2013.09.005Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, Louisiana, 2010, 1–8. https://doi.org/10.1109/GCE.2010.5676129
DOI: https://doi.org/10.1109/GCE.2010.5676129Nagaty, H.F. (1942) Trematodes of fishes from the Red Sea. Part 3. On seven new allocreadiid species. Publications of the Marine Biological Station, Ghardaqa, Red Sea, 4, 1–27.
Nguyen, L.-T., Schmidt, H.A., Von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300
DOI: https://doi.org/10.1093/molbev/msu300Olson, P.D., Cribb, T.H., Tkach, V.V., Bray, R.A. & Littlewood, D.T.J. (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755. https://doi.org/10.1016/S0020-7519(03)00049-3
DOI: https://doi.org/10.1016/S0020-7519(03)00049-3Overstreet, R.M. (1969) Digenetic trematodes of marine teleost fishes from Biscayne Bay, Florida. Tulane Studies in Zoology and Botany, 15, 119–176.
Pérez-Ponce de León, G., Garcia-Prieto, L. & Mendoza-Garfias, B. (2007) Trematode parasites (Platyhelminthes) of wildlife vertebrates in Mexico. Zootaxa, 1534 (1), 1–247. https://doi.org/10.11646/zootaxa.1534.1.1
DOI: https://doi.org/10.11646/zootaxa.1534.1.1Pleijel, F., Jondelius, U., Norlinder, E., Nygren, A., Oxelman, B., Schander, C., Sundberg, P. & Thollesson, M. (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371. https://doi.org/10.1016/j.ympev.2008.03.024
DOI: https://doi.org/10.1016/j.ympev.2008.03.024Poulin, R., Presswell, B. & Jorge, F. (2020) The state of fish parasite discovery and taxonomy: a critical assessment and a look forward. International Journal for Parasitology, 50, 733–742. https://doi.org/10.1016/j.ijpara.2019.12.009
DOI: https://doi.org/10.1016/j.ijpara.2019.12.009Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
DOI: https://doi.org/10.1093/sysbio/sys029Sambrook, J. & Russell, D.W. (2001) Molecular Cloning: A Laboratory Manual. Vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 112 pp.
Saoud, M.F.A. & Ramadan, M.M. (1985) Studies on the trematodes of the genus Enenterum Linton, 1910 (Opecoelidae) and the genus Pseudocreadium Layman, 1930 (Lepocreadiidae) from some Red Sea fish. Qatar University Science Bulletin, 5, 233–253.
Sogandares-Bernal, F. (1959) Digenetic trematodes of marine fishes from the Gulf of Panama and Bimini, British West Indies. Tulane Studies in Zoology, 7, 69–117.
Spalding, M.D., Fox, H.E., Allen, G.R., Davidson, N., Ferdana, Z.A., Finlayson, M.A.X., Halpern, B.S., Jorge, M.A., Lombana, A.L. & Lourie, S.A. (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience, 57, 573–583. https://doi.org/10.1641/B570707
DOI: https://doi.org/10.1641/B570707Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
DOI: https://doi.org/10.1093/bioinformatics/btu033Towns, J., Cockerill, T., Dahan, M., Foster, I., Gaither, K., Grimshaw, A., Hazlewood, V., Lathrop, S., Lifka, D. & Peterson, G.D. (2014) XSEDE: accelerating scientific discovery. Computing in Science & Engineering, 16, 62–74. https://doi.org/10.1109/MCSE.2014.80
DOI: https://doi.org/10.1109/MCSE.2014.80Winter, H.A. (1957) Tremátodos de peces marinos de aguas mexicanas XII. Dos géneros de digéneos (Lepocreadiidae), incluyendo una nueva especie procedente de Kyphosus elegans (Peters) de las Islas Tres Marías, en el Oceano Pacífico. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología, 27, 403–413.
Yamaguti, S. (1958) Systema Helminthum. Vol. I. The Digenetic Trematodes of Vertebrates. Part I. Interscience Publishers, Inc., New York and London, 979 pp.
Yamaguti, S. (1970) Digenetic Trematodes of Hawaiian fishes. Keigaku Publishing Co., Tokyo, 436 pp.