Abstract
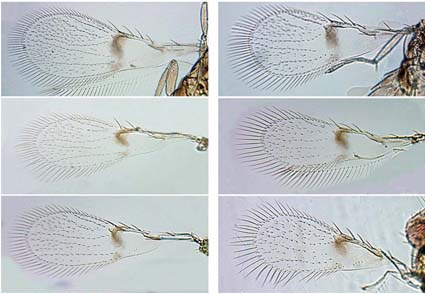
The present study evaluated the taxonomy, morphology, and molecular variation of the egg parasitoids Paracentrobia subflava developing within eggs of the corn leafhopper Dalbulus maidis from Mexico, and Paracentrobia tapajosae developing within eggs of Tapajosa rubromarginata and eggs of D. maidis from Argentina. The parasitoids from the different host species were found to have a significant difference in body size and morphology of head, wings, ovipositor, and chaetotaxy. On the other hand, geometric morphometric analysis of the male genitalia showed no difference between parasitoids emerged from T. rubromarginata and D. maidis. Additionally, the COI and ITS2 molecular markers demonstrated that the parasitoids emerging from these two different hosts cluster into a single clade. This new information suggests the placement of P. tapajosae syn. nov. as a junior synonym of P. subflava.
References
Adams, D.C., Rohlf, F.J. & Slice, D.E. (2013) A field comes of age: geometric morphometrics in the 21st century. Hystrix, 24, 7–14.
Adams, D.C., Collyer, M.L., Kaliontzopoulou, A. & Balken, E. (2021) Geomorph: Software for geometric morphometric analyses. R package version 3.3.2. Available from: https://cran.r-project.org/package=geomorph (accessed 12 March 2022)
Alvarez, J.M. & Hoy, M.A. (2002) Evaluation of the ribosomal ITS2 DNA sequences in separating closely related populations of the parasitoid Ageniaspis (Hymenoptera: Encyrtidae). Annals of the Entomological Society of America, 95, 250–256. https://doi.org/10.1603/0013-8746(2002)095[0250:EOTRID]2.0.CO;2
Bookstein, F.L. (1991) Morphometric tools for landmark data: Geometry and Biology. Cambridge University Press, New York, 435 pp. https://doi.org/10.1017/CBO9780511573064
Boulesteix, A.L. (2005) A note on between-group PCA. International Journal of Pure and Applied Mathematics, 19, 359–366.
Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012) JModelTest 2: More models, new heuristics and parallel computing. Nature Methods, 9, 772. https://doi.org/10.1038/nmeth.2109
Eggleton, P. & Gaston, K. J. (1990) " Parasitoid " Species and Assemblages : Convenient Definitions or Misleading Compromises ?. Oikos, 59, 417–421. [https://www.jstor.org/stable/3545155]
Girault, A.A. (1911) Descriptions of nine new genera of the chalcidoid family Trichogrammatidae. Transactions of the American Entomological Society, 37, 1–43. https://doi.org/10.4039/Ent43168-5
Guzmán-Larralde, A.J., Suaste-Dzul, A.P., Gallou, A. & Peña-Carrillo, K.I. (2017) DNA recovery from microhymenoptera using six non-destructive methodologies with considerations for subsequent preparation of museum slides. NRC Research Press, 60, 85–91. https://doi.org/10.1139/gen-2015-0172
Goodall, C. (1991) Procrustes methods in the statistical analysis of shape. Journal of the Royal Statistical Society, Series B (Methodological), 53, 285–339. https://doi.org/10.1111/j.2517-6161.1991.tb01825.x
Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. The Nucleic Acids Symposium Series, 41, 95–98.
Harvey, J.A. (2005) Factors affecting the evolution of development strategies in parasitoid wasps: The importance of functional constraints and incorporating complexity. Entomologia Experimentalis et Applicata, 117, 1–13. https://doi.org/10.1111/j.1570-7458.2005.00348.x
Heraty, J.M., Woolley, J.B., Hopper, K.R., Hawks, D.L., Kim, J.W. & Buffington, M. (2007) Molecular phylogenetics and reproductive incompatibility in a complex of cryptic species of aphid parasitoids. Molecular Phylogenetics and Evolution, 45, 480–493. https://doi.org/10.1016/j.ympev.2007.06.021
Johnson, N.F., Rawlins, J.E. & Pavuk, D.M. (1987) Host-related antennal variation in the polyphagous egg parasite Telenomus alsophilae (Hymenoptera: Scelionidae). Systematic Entomology, 12, 437–447. https://doi.org/10.1111/j.1365-3113.1987.tb00216.x
Katoh, K., Misawa, K., Kuma, K.I. & Miyata, T. (2002) MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066. https://doi.org/10.1093/nar/gkf436
Lenicov, D.R., Virla, E. & Manca, M. (1998) Difusión de Tapajosa rubromarginata (Homptera: Cicadellidae) sobre cultivos cerealeros de la Argentina. Revista de la Sociedad Entomologica de Argentina, 57, 1-4.
Luft Albarracin, E., Triapitsyn, S.V. & Virla, E.G. (2017) Egg Parasitoid Complex of the Corn Leafhopper, Dalbulus maidis (DeLong) (Hemiptera: Cicadellidae), in Argentina. Neotropical Entomology, 46, 666–677. https://doi.org/10.1007/s13744-017-0535-x
Mitteroecker, P. & Gunz, P. (2009) Advances in geometric morphometrics. Evolutionary Biology, 36, 235–247. https://doi.org/10.1007/s11692-009-9055-x
Mitteroecker, P. & Bookstein, F. (2011) Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evolutionary Biology, 38, 100–114. https://doi.org/10.1007/s11692-011-9109-8
Moya-Raygoza, G., Luft Albarracin, E. & Virla, E.G. (2012) Diversity of egg parasitoids attacking Dalbulus maidis (Hemiptera: Cicadellidae) population at low and high elevation sites in Mexico and Argentina. Florida Entomologist, 95, 105–112. https://doi.org/10.1653/024.095.0117
Moya-Raygoza, G., Renteria, C., Luft Albarracin, E. & Virla, E.G. (2014) Egg parasitoids of the leafhoppers Dalbulus maidis and Dalbulus elimatus (Hemiptera: Cicadellidae) in two maize habitats. Florida Entomologist, 97, 309–312. https://doi.org/10.1896/054.097.0148
Moya-Raygoza, G. & Triapitsyn, S.V. (2015) Egg parasitoids (Hymenoptera: Mymaridae and Trichogrammatidae) of Dalbulus quinquenotatus (Hemiptera: Cicadellidae), with description of a new species of Anagrus (Mymaridae) from Mexico. Annals of the Entomological Society of America, 108, 289–298. https://doi.org/10.1093/aesa/sav025
Moya-Raygoza, G. & Triapitsyn, S.V. (2017) Egg parasitoids of Dalbulus maidis on Wild Teosintes in Mexico. Southwestern Entomologist, 42, 691–700. https://doi.org/10.3958/059.042.0307
Nagarkatti, S. & Nagaraja, H. (1971) Redescriptions of some known species of Trichogramma (Hym., Trichogrammatidae), showing the importance of the male genitalia as a diagnostic character. Bulletin of Entomological Research, 61, 13–31. https://doi.org/10.1017/S0007485300057412
Nault, L.R. (1980) Maize bushy stunt and corn stunt: a comparison of disease symptoms, pathogen host ranges, and vectors. Phytopathology, 70, 659–662. https://doi.org/10.1094/Phyto-70-659
Nault, L.R. (1990) Evolution of an insect pest: maize and the corn leafhopper, a case study. Maydica, 35, 165–175.
Owen, A.K., George, J., Pinto, J.D. & Heraty, J.M. (2007) A molecular phylogeny of the Trichogrammatidae (Hymenoptera: Chalcidoidea), with an evaluation of the utility of their male genitalia for higher level classification. Systematic Entomology, 32, 227–251. https://doi.org/10.1111/j.1365-3113.2006.00361.x
Pigliucci, M. (2005) Evolution of phenotypic plasticity: Where are we going now? Trends in Ecology and Evolution, 20, 481–486. https://doi.org/10.1016/j.tree.2005.06.001
Pinto, J.D., Velten, R.K., Platner, G.R. & Oatman, E.R. (1989) Phenotypic plasticity and taxonomic characters in Trichogramma (Hymenoptera: Trichogrammatidae). Annals of the Entomological Society of America, 82, 414–425. https://doi.org/10.1093/aesa/82.4.414
Pinto, J.D. (1997) Taxonomia de Trichogrammatidae (Hymenoptera) com ênfase nos gêneros que parasitam Lepidoptera. In: Parra, J.R.P. & Zucchi, R.A. (Eds.), Trichogramma e o controle biológico aplicado. FEALQ, Piracicaba, pp. 13–39.
Pinto, J.D. (1999) Systematics of the North American species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae). Entomological Society of Washington Memoirs, 22, 1–287.
Pinto, J.D. (2006) A review of the new world genera of Trichogrammatidae (Hymenoptera). Journal of Hymenoptera Research, 15, 38–163.
Querino, R.B., De Moraes, R.C.B. & Zucchi, R.A. (2002) Relative warp analysis to study morphological variations in the genital capsule of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Neotropical Entomology, 31, 217–224. https://doi.org/10.1590/S1519-566X2002000200007
Querino, R.B. & Zucchi, R.A. (2003) Caracterização Morfológica de dez espécies de Trichogramma (Hymenoptera: Trichogrammatidae) registradas na América do Sul. Neotropical Entomology, 32, 597–613. https://doi.org/10.1590/S1519-566X2003000400010
Querino, R.B., Zucchi, R.A. & Pinto, J.D. (2010) Systematics of the Trichogrammatidae (Hymenoptera : Chalcidoidea) with a focus on the genera attacking Lepidoptera. In: Cônsoli, F.L., Parra, J.R.P. & Zucchi, R.A (Eds.), Egg parasitoids in agroecosystems with emphasis on Trichogramma. Dordrecht, Springer, pp. 191–218. https://doi.org/10.1007/978-1-4020-9110-0
Querino, R.B., Meneses, A.R., Luft Albarracin, E., Oliveira, C.M. & Triapitsyn, S.V. (2017) Biological control de Dalbulus maidis in Brazil: An overview of the parasitoids. Embrapa, 1, 121–140.
Rohlf, F.J. (2017) tpsDig2. Version 2.22. Department of Ecology and Evolution. State University of New York, Stony Brook. Available from: https://life.bio.sunysb.edu/morph/ (accessed 12 March 2022)
Rohlf, F.J. (2018) tpsUtil. Version 1.76. Department of Ecology and Evolution. State University of New York, Stony Brook. Available from: https://life.bio.sunysb.edu/morph/ (accessed 12 March 2022)
Rohlf, F.J. & Marcus, L.S. (1993) A revolution in morphometrics. Trends in Ecology and Evolution, 8, 128–132. https://doi.org/10.1016/0169-5347(93)90024-J
Salt, G. (1937) The egg-parasite of Sialis lutaria: A study of the influence of the host upon a dimorphic parasite. Parasitology, 29, 539–553. https://doi.org/10.1017/S0031182000025063
Salt, G. (1941) The effects of hosts upon their insect parasites. Biological Reviews, 16, 239–264. https://doi.org/10.1111/j.1469-185X.1941.tb01103.x
Schlager, S. (2017) Morpho and Rvcg - Shape analysis in R. In: (Zheng, G., Li, S. & Szekely, G. (Eds.), Statistical shape and deformation analysis: Methods, implementation and applications. Academic Press, London, pp. 217–256. https://doi.org/10.1016/B978-0-12-810493-4.00011-0
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H. & Flook, P. (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primer. Annals of Entomology Society of America, 87, 651–701. https://doi.org/10.1093/aesa/87.6.651
Slice, D.E. (2007) Geometric morphometrics. Annual Review of Anthropology, 36, 261–281. https://doi.org/10.1146/annurev.anthro.34.081804.120613
Stamatakis, A. (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Stouthamer, R., Hu, J., Van Kan, F.J.P.M., Platner, G.R. & Pinto J.D. (1999) The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for dis- tinguishing sibling species of Trichogramma. BioControl, 43, 421–440. https://doi.org/10.1023/A:1009937108715
Torres-Moreno, R., Victor, J. & Moya-Raygoza, G. (2021) Morphological variation of the parasitic wasp Paracentrobia subflava (Hymenoptera: Trichogrammatidae) emerged from different leafhopper species. Zoologischer Anzeiger, 294, 20-27. https://doi.org/10.1016/j.jcz.2021.07.007
Triapitsyn, S.V., Rugman-Jones, P.F., Tretiakov, P.S., Luft Albarracin, E., Moya-Raygoza, G. & Querino, R.B. (2018) Molecular, morphological, and biological differentiation between Anagrus virlai sp. n., an egg parasitoid of the corn leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) in the New World, and Anagrus incarnatus from the Palaearctic region (Hymenoptera: Mymaridae). Neotropical Entomology, 48, 87–97. https://doi.org/10.1007/s13744-018-0606-7
Triapitsyn, S.V., Rugman-Jones, P.F., Tretiakov, P.S., Daane, K.M. & Wilson, H. (2020) Reassessment of molecular and morphological variation within the Anagrus atomus species complex (Hymenoptera: Mymaridae): egg parasitoids of leafhoppers (Hemiptera: Cicadellidae) in Europe and North America. Journal of Natural History, 54, 1735–1758. https://doi.org/10.1080/00222933.2020.1827073
Virla, E.G. (1999) Aportes acerca de la bionomía de Paracentrobia (P.) subflava (Hymenoptera: Trichogrammatidae), parasitoide de Hemípteros Cicadeloideos argentinos. Revista de la Sociedad Entomológica Argentina, 58 (3–4), 17–22.
Virla, E.G., Díaz, C.G., Carpane, P., Laguna, I.G., Ramallo, J., Gerónimo Gómez, L. & Giménez Pecci, M.P. (2004) Evaluación preliminar de la disminución en la producción de maíz causada por el " Corn stunt spiroplasma " (CSS) en Tucumán , Argentina. Boletín de Sanidad Vegetal. Plagas, 30, 403–413.
Virla, E.G., Luft Albarracin, E. & Moya-Raygoza, G. (2009a) Egg parasitoids of Dalbulus maidis (Hemiptera: Cicadellidae) in Jalisco State, Mexico. Florida Entomologist, 92, 508–510. https://doi.org/10.1653/024.092.0316
Virla, E.G., Luft Albarracin, E., Triapitsyn, S.V., Viggiani, G. & Logarzo, G.A. (2009b) Description and biological traits of a new species of Paracentrobia (Hymenoptera: Trichogrammatidae), an egg parasitoid of the sharpshooter Tapajosa rubromarginata (Hemiptera: Cicadellidae) in Argentina. Studies on Neotropical Fauna and Environment, 44, 47–53.
https://doi.org/10.1080/01650520902826831
Zelditch, M.L., Swiderski, D. & Sheets, H.D. (2012) Geometric morphometrics for biologists: A primer. Second Edition. Academic Press, Amsterdam, 488 pp.