Abstract
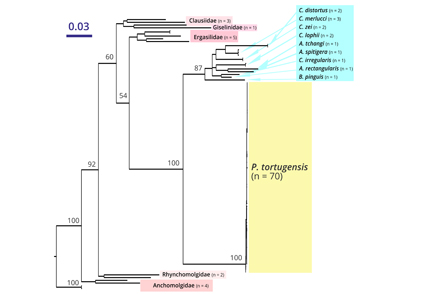
Previous work, using morphological characters, identified a generalist copepod parasite (Pharodes tortugensis) at high prevalence on two common gobies (Coryphopterus glaucofraenum and C. dicrus) in the British Virgin Islands (BVI). DNA barcoding subsequently revealed C. glaucofraenum to be three morphologically similar species (C. glaucofraenum, C. venezuelae and C. tortugae), casting doubt on host identities in the BVI and the classification of the parasite as a single species. Mitochondrial cytochrome c oxidase subunit I (COI) data from 67 gobies in the BVI showed that, in addition to C. dicrus, host gobies were a mix of C. glaucofraenum and C. venezuelae, while C. tortugae was unexpectedly absent from the study area. COI data (n = 70) indicated that the copepod infecting all three hosts was a single species, almost certainly P. tortugensis. The pharodes-coryphopterus interaction has a strong impact on host dynamics in the BVI, and a revised understanding of these dynamics must account for any differences among the three newly confirmed hosts in transmission of, and susceptibility to, the shared parasite. No other infected hosts were discovered at our sites, but P. tortugensis is reportedly widespread and infects 12 additional host species elsewhere. Further DNA barcoding is thus needed to test whether P. tortugensis is truly a widespread generalist, or instead represents a group of more specialized cryptic species.
References
Baldwin, C., Weigt, L., G Smith, D. & H Mounts, J. (2009) Reconciling Genetic Lineages with Species in Western Atlantic Coryphopterus (Teleostei: Gobiidae). Smithsonian Contributions to the Marine Sciences, 38, 111–138.
Baldwin, C.C. & Robertson, D.R. (2015) A new, mesophotic Coryphopterus goby (Teleostei, Gobiidae) from the southern Caribbean, with comments on relationships and depth distributions within the genus. ZooKeys, 2015, 123–142. https://doi.org/10.3897/zookeys.513.9998
Banks, J.C. & Paterson, A.M. (2005) Multi-host parasite species in cophylogenetic studies. International journal for parasitology, 35, 741–746. https://doi.org/10.1016/j.ijpara.2005.03.003
Besansky, N.J. (1999) Complexities in the analysis of cryptic taxa within the genus Anopheles. Parassitologia, 41, 97–100.
Bickford, D., Lohman, D.J., Sodhi, N.S., Ng, P.K.L., Meier, R., Winker, K., Ingram, K.K. & Das, I. (2007) Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution, 22, 148–155. https://doi.org/10.1016/j.tree.2006.11.004
Bland, M. (2013) Detecting a single event. In: British Standards Institution Study Day. British Standards Institution, York, pp. 4.
Böhlke, J.E. & Robins, C.R. (1960) A Revision of the Gobioid Fish Genus Coryphopterus. Proceedings of the Academy of Natural Sciences of Philadelphia, 112, 103–128.
Boxshall, G. & Hayes, P. (2019) Biodiversity and Taxonomy of the Parasitic Crustacea. In: Smit, N. J., Bruce, N.L. & Hadfield, K.A. (Eds.), Parasitic Crustacea: State of Knowledge and Future Trends. Zoological Monographs. Springer International Publishing, Cham, pp. 73–134. https://doi.org/10.1007/978-3-030-17385-2_3
Bucklin, A., Ortman, B.D., Jennings, R.M., Nigro, L.M., Sweetman, C.J., Copley, N.J., Sutton, T. & Wiebe, P.H. (2010) A “Rosetta Stone” for metazoan zooplankton: DNA barcode analysis of species diversity of the Sargasso Sea (Northwest Atlantic Ocean). Deep Sea Research Part II: Topical Studies in Oceanography, 57, 2234–2247. https://doi.org/10.1016/j.dsr2.2010.09.025
Cervigón, F. (1994) Complemento lll Los Peces Marinos de Venezuela. 2ème Édition. Fundación Científica Los Roques, Caracas, 499 pp.
Costello, M.J. (2016) Parasite Rates of Discovery, Global Species Richness and Host Specificity. Integrative and Comparative Biology, 56, 588–599. https://doi.org/10.1093/icb/icw084
Dobson, A. (2004) Population dynamics of pathogens with multiple host species. American Naturalist, 164, S64–S78. https://doi.org/10.1086/424681
Finley, R. & Forrester, G. (2003) Impact of ectoparasites on the demography of a small reef fish. Marine Ecology Progress Series 248, 305–309. https://doi.org/10.3354/meps248305
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Forrester, G., Harmon, L., Helyer, J., Holden, W. & Karis, R. (2011) Experimental evidence for density-dependent reproductive output in a coral reef fish. Population Ecology, 53, 155–163. https://doi.org/10.1007/s10144-010-0225-6
Forrester, G.E. (1995) Strong density-dependent survival and recruitment regulate the abundance of a coral reef fish. Oecologia, 103, 275–282. https://doi.org/10.1007/BF00328615
Forrester, G.E. (1999) The influence of adult density on larval settlement in a coral reef fish Coryphopterus glaucofraenum. Coral Reefs, 18, 85–89. https://doi.org/10.1007/s003380050159
Forrester, G.E., Chille, E., Nickles, K. & Reed, K. (2019) Behavioural mechanisms underlying parasite-mediated competition for refuges in a coral reef fish. Scientific Reports, 9, 15487. https://doi.org/10.1038/s41598-019-52005-y
Forrester, G.E. & Finley, R.J. (2006) Parasitism and a Shortage of Refuges Jointly Mediate the Strength of Density Dependence in a Reef Fish. Ecology, 87, 1110–1115. https://doi.org/10.1890/0012-9658(2006)87[1110:PAASOR]2.0.CO;2
Forrester, G.E. & Steele, M.A. (2000) Variation in the Presence and Cause of Density-Dependent Mortality in Three Species of Reef Fishes. Ecology 81, 2416–2427. https://doi.org/10.1890/0012-9658(2000)081[2416:VITPAC]2.0.CO;2
Forrester, G.E. & Steele, M.A. (2004) Predators, Prey Refuges, and the Spatial Scaling of Density-Dependent Prey Mortality. Ecology, 85, 1332–1342. https://doi.org/10.1890/03-0184
Garzón-Ferreira, J. & Arturo Acero, P. (1990) Redescription of Coryphopterus tortugae (Jordan) (Osteichthyes: Gobiidae), A Valid Species of Goby from the Western Atlantic. Gulf of Mexico Science, 11 (2). [published online] https://doi.org/10.18785/negs.1102.02
Goedknegt, M.A., Thieltges, D.W., van der Meer, J., Wegner, K.M. & Luttikhuizen, P.C. (2018) Cryptic invasion of a parasitic copepod: Compromised identification when morphologically similar invaders co-occur in invaded ecosystems. PLoS ONE, 13, e0193354. https://doi.org/10.1371/journal.pone.0193354
Greenfield, D.W. & Johnson, R.K. (1999) Assemblage structure and habitat associations of western Caribbean gobies (Teleostei: Gobiidae). Copeia, 1999 (2), 251–266. https://doi.org/10.2307/1447470
Haydon (2002) Identifying Reservoirs of Infection: A Conceptual and Practical Challenge. Emerging Infectious Diseases, 8, 1468–1473. https://doi.org/10.3201/eid0812.010317
Ho, J.-S. (1971) Pharodes Wilson, 1935, a genus of cyclopoid copepods (Pharodidae) parasitic on marine fishes. Journal of Natural History, 5, 349–359. https://doi.org/10.1080/00222937100770261
Holt, R.D., Dobson, A.P., Begon, M., Bowers, R.G. & Schauber, E.M. (2003) Parasite establishment in host communities. Ecology Letters, 6, 837–842. https://doi.org/10.1046/j.1461-0248.2003.00501.x
Horton, T., Kroh, A., Ahyong, S., Bailly, N., Boyko, C.B., Brandão, S.N., Gofas, S., Hooper, J.N.A., Hernandez, F., Holovachov, O., Mees, J., Molodtsova, T.N., Paulay, G., Decock, W., Dekeyzer, S., Poffyn, G., Vandepitte, L., Vanhoorne, B., Adlard, R., Agatha, S., Ahn, K.J., Akkari, N., Alvarez, B., Anderberg, A., Anderson, G., Angel, M.V., Antic, D., Arango, C., Artois, T., Atkinson, S., Auffenberg, K., Baldwin, B.G., Bank, R., Barber, A., Barbosa, J.P., Bartsch, I., Bellan-Santini, D., Bergh, N., Bernot, J., Berta, A., Bezerra, T.N., Bieler, R., Blanco, S., Blasco-Costa, I., Blazewicz, M., Bock, P., Bonifacino de León, M., Böttger-Schnack, R., Bouchet, P., Boury-Esnault, N., Boxshall, G., Bray, R., Bruce, N.L., Cairns, S., Calvo Casas, J., Carballo, J.L., Cárdenas, P., Carstens, E., Chan, B.K., Chan, T.Y., Cheng, L., Churchill, M., Coleman, C.O., Collins, A.G., Collins, G.E., Corbari, L., Cordeiro, R., Cornils, A., Coste, M., Costello, M.J., Crandall, K.A., Cremonte, F., Cribb, T., Cutmore, S., Dahdouh-Guebas, F., Daly, M., Daneliya, M., Dauvin, J.C., Davie, P., De Broyer, C., De Grave, S., de Mazancourt, V., de Voogd, N.J., Decker, P., Decraemer, W., Defaye, D., d’Hondt, J.L., Dippenaar, S., Dohrmann, M., Dolan, J., Domning, D., Downey, R., Ector, L., Eisendle-Flöckner, U., Eitel, M., Encarnação, S.C. d., Enghoff, H., Epler, J., Ewers-Saucedo, C., Faber, M., Figueroa, D., Finn, J., Fišer, C., Fordyce, E., Foster, W., Frank, J.H., Fransen, C., Freire, S., Furuya, H., Galea, H., Gao, T., Garcia-Alvarez, O., Garcia-Jacas, N., Garic, R., Garnett, S., Gasca, R., Gaviria-Melo, S., Gerken, S., Gibson, D., Gibson, R., Gil, J., Gittenberger, A., Glasby, C., Glover, A., Gómez-Noguera, S.E., González-Solís, D., Gordon, D., Gostel, M., Grabowski, M., Gravili, C., Guerra-García, J.M.., Guidetti, R., Guiry, M.D., Gutierrez, D., Hadfield, K.A., Hajdu, E., Hallermann, J., Hayward, B.W., Heiden, G., Hendrycks, E., Herbert, D., Herrera Bachiller, A., Ho, J. s., Hodda, M., Høeg, J., Hoeksema, B., Houart, R., Hughes, L., Hyžný, M., Iniesta, L.F.M., Iseto, T., Ivanenko, S., Iwataki, M., Janssen, R., Jarms, G., Jaume, D., Jazdzewski, K., Jersabek, C.D., Jóźwiak, P., Kabat, A., Kantor, Y., Karanovic, I., Karthick, B., Katinas, L., Kim, Y.H., King, R., Kirk, P.M., Klautau, M., Kociolek, J.P., Köhler, F., Kolb, J., Kotov, A., Kremenetskaia, A., Kristensen, R.M., Kulikovskiy, M., Kullander, S., Kupriyanova, E., Lambert, G., Lazarus, D., Le Coze, F., LeCroy, S., Leduc, D., Lefkowitz, E.J., Lemaitre, R., Lichter-Marck, I.H., Lindsay, D., Liu, Y., Loeuille, B., Lörz, A.N., Lowry, J., Ludwig, T., Lundholm, N., Macpherson, E., Madin, L., Mah, C., Mamo, B., Mamos, T., Manconi, R., Mapstone, G., Marek, P.E., Marshall, B., Marshall, D.J., Martin, P., Mast, R., McFadden, C., McInnes, S.J., Meidla, T., Meland, K., Melo da Silva, D.C., Merrin, K.L., Messing, C., Mills, C., Moestrup, Ø., Mokievsky, V., Monniot, F., Mooi, R., Morandini, A.C., Moreira da Rocha, R., Morrow, C., Mortelmans, J., Mortimer, J., Musco, L., Nesom, G., Neubauer, T.A., Neubert, E., Neuhaus, B., Ng, P., Nguyen, A.D., Nielsen, C., Nishikawa, T., Norenburg, J., O’Hara, T., Opresko, D., Osawa, M., Osigus, H.J., Ota, Y., Páll-Gergely, B., Panero, J.L., Pasini, E., Patterson, D., Paxton, H., Pelser, P., Peña-Santiago, R., Perrier, V., Petrescu, I., Pica, D., Picton, B., Pilger, J.F., Pisera, A.B., Polhemus, D., Poore, G.C., Potapova, M., Pugh, P., Read, G., Reich, M., Reimer, J.D., Reip, H., Reuscher, M., Reynolds, J.W., Richling, I., Rimet, F., Ríos, P., Rius, M., Rodríguez, E., Rogers, D.C., Roque, N., Rosenberg, G., Rützler, K., Sabbe, K., Saiz-Salinas, J., Sala, S., Santagata, S., Santos, S., Sar, E., Satoh, A., Saucède, T., Schatz, H., Schierwater, B., Schilling, E., Schmidt-Rhaesa, A., Schneider, S., Schönberg, C., Schuchert, P., Senna, A.R., Serejo, C., Shaik, S., Shamsi, S., Sharma, J., Shear, W.A., Shenkar, N., Shinn, A., Short, M., Sicinski, J., Sierwald, P., Simmons, E., Sinniger, F., Sivell, D., Sket, B., Smit, H., Smit, N., Smol, N., Souza-Filho, J.F.., Spelda, J., Sterrer, W., Stienen, E., Stoev, P., Stöhr, S., Strand, M., Suárez-Morales, E., Summers, M., Suppan, L., Susanna, A., Suttle, C., Swalla, B.J., Taiti, S., Tanaka, M., Tandberg, A.H., Tang, D., Tasker, M., Taylor, J., Taylor, J., Tchesunov, A., Temereva, E., ten Hove, H., ter Poorten, J.J., Thomas, J.D., Thuesen, E.V., Thurston, M., Thuy, B., Timi, J.T., Timm, T., Todaro, A., Turon, X., Tyler, S., Uetz, P., Urbatsch, L., Uribe-Palomino, J., Urtubey, E., Utevsky, S., Vacelet, J., Vachard, D., Vader, W., Väinölä, R., Van de Vijver, B., van der Meij, S.E., van Haaren, T., van Soest, R.W., Vanreusel, A., Venekey, V., Vinarski, M., Vonk, R., Vos, C., Walker-Smith, G., Walter, T.C., Watling, L., Wayland, M., Wesener, T., Wetzel, C.E., Whipps, C., White, K., Wieneke, U., Williams, D.M., Williams, G., Wilson, R., Witkowski, A., Witkowski, J., Wyatt, N., Wylezich, C., Xu, K., Zanol, J., Zeidler, W. & Zhao, Z. (2020) World Register of Marine Species (WoRMS). World Register of Marine Species (WoRMS). Available from: http://www.marinespecies.org (accessed 4 December 2020)
Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108
de León, G.P.-P. & Nadler, S.A. (2010) What We Don’t Recognize Can Hurt Us: A Plea for Awareness About Cryptic Species. Journal of Parasitology, 96, 453–464. https://doi.org/10.1645/GE-2260.1
Marshall, E. (2005) Taxonomy: Will DNA Bar Codes Breathe Life Into Classification? Science, 307, 1037–1037. https://doi.org/10.1126/science.307.5712.1037
McDonald, J.H. (2014) Handbook of Biological Statistics. 3rd Edition. Sparky House Publishing, Baltimore, Maryland, 299 pp.
Nadler, S.A. & De Leon, G.P.-P. (2011) Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology, 138, 1688. https://doi.org/10.1017/S003118201000168X
Nguyen, L.-T., Schmidt, H.A., von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Molecular Biology and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300
Noble, E.R. (1989) Parasitology: the biology of animal parasites. Lea & Febiger, Philadelphia, Pennsylvania, 574 pp.
Østergaard, P., Boxshall, G.A. & Quicke, D.L. (2003) Phylogeny within the Chondracanthidae (Poecilostomatoida, Copepoda). Zoologica Scripta, 32, 299–319. https://doi.org/10.1046/j.1463-6409.2003.00113.x
Paterson, A.M. & Poulin, R. (1999) Have chondracanthid copepods co-speciated with their teleost hosts? Systematic Parasitology, 44, 79–85. https://doi.org/10.1023/A:1006255822947
Petrik-Finley, R.J.M. (2005) The impact of a parasitic gill copepod on the demography of a reef fish host. Ph.D., University of Rhode Island, Kingston, Rhode Island. [unknown pagination]
Poulin, R. (1992) Determinants of host-specificity in parasites of freshwater fishes. International journal for parasitology, 22, 753–758. https://doi.org/10.1016/0020-7519(92)90124-4
Poulin, R. (2014) Parasite biodiversity revisited: frontiers and constraints. International Journal for Parasitology, 44, 581–589. https://doi.org/10.1016/j.ijpara.2014.02.003
Poulin, R. & Keeney, D.B. (2008) Host specificity under molecular and experimental scrutiny. Trends in Parasitology, 24, 24–28. https://doi.org/10.1016/j.pt.2007.10.002
Poulin, R., Krasnov, B.R. & Mouillot, D. (2011) Host specificity in phylogenetic and geographic space. Trends in Parasitology, 27, 355–361. https://doi.org/10.1016/j.pt.2011.05.003
Robertson, D.R. & Van Tassell, J.L. (2019) Shorefishes - Homepage. Fishes: Greater Caribbean. A guide to shorefishes of the Caribbean and adjacent areas. Version 2.0. Available from: https://biogeodb.stri.si.edu/caribbean/en/pages (accessed 21 September 2020)
Samhouri, J.F., Vance, R.R., Forrester, G.E. & Steele, M.A. (2009) Musical chairs mortality functions: density-dependent deaths caused by competition for unguarded refuges. Oecologia, 160, 257–265. https://doi.org/10.1007/s00442-009-1307-z
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P. & Cardona, A. (2012) Fiji: an open-source platform for biological-image analysis. Nature Methods, 9, 676–682. https://doi.org/10.1038/nmeth.2019
Smith, M.A., Woodley, N.E., Janzen, D.H., Hallwachs, W. & Hebert, P.D. (2006) DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proceedings of the National Academy of Sciences, 103, 3657–3662. https://doi.org/10.1073/pnas.0511318103
Thacker, C. & Cole, K. (2002) Phylogeny and evolution of the gobiid genus Coryphopterus. Bulletin of Marine Science, 70, 837–850.
Trontelj, P. & Fišer, C. (2010) Perspectives: Cryptic species diversity should not be trivialised. Systematics and Biodiversity, 7, 1–3. https://doi.org/10.1017/S1477200008002909
Vance, R.R., Steele, M.A. & Forrester, G.E. (2010) Using an individual-based model to quantify scale transition in demographic rate functions: Deaths in a coral reef fish. Ecological Modelling, 221, 1907–1921. https://doi.org/10.1016/j.ecolmodel.2010.04.014
Victor, B. (2008) Redescription of Coryphopterus tortugae (Jordan) and a new allied species Coryphopterus bol (Perciformes: Gobiidae: Gobiinae) from the tropical western Atlantic Ocean. Journal of the Ocean Science Foundation, 1, 1–19.
Victor, B.C. (2007) Coryphopterus kuna, a new goby (Perciformes : Gobiidae : Gobiinae) from the western Caribbean, with the identification of the late larval stage and an estimate of the pelagic larval duration. Zootaxa, 1526 (1), 51–61. https://doi.org/10.11646/zootaxa.1526.1.3
Victor, B.C. (2015) Western Atlantic Coryphopterus gobies. Western Atlantic Coryphopterus gobies. Available from: http://www.coralreeffish.com/gobiidae2adult.html (accessed 21 September 2020)
Volk, D.R., Konvalina, J.D., Floeter, S.R., Ferreira, C.E.L. & Hoffman, E.A. (2020) Going against the flow: Barriers to gene flow impact patterns of connectivity in cryptic coral reef gobies throughout the western Atlantic. Journal of Biogeography, 48 (2), 427–439. https://doi.org/10.1111/jbi.14010
Walters, V. (1953) Diocus frigidus (Copepoda; Chondracanthidae) parasitic in eelpouts at Pt. Barrow, Alaska, with notes on the species of Diocus and a revision of the diagnosis of Pharodes. The Journal of Parasitology, 39, 169–177. https://doi.org/10.2307/3274113
Ward, R.D., Hanner, R. & Hebert, P.D. (2009) The campaign to DNA barcode all fishes, FISH-BOL. Journal of fish biology, 74, 329–356. https://doi.org/10.1111/j.1095-8649.2008.02080.x
Ward, R.D., Zemlak, T.S., Innes, B.H., Last, P.R. & Hebert, P.D.N. (2005) DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society B: Biological Sciences, 360, 1847–1857. https://doi.org/10.1098/rstb.2005.1716
Westram, A.M., Baumgartner, C., Keller, I. & Jokela, J. (2011) Are cryptic host species also cryptic to parasites? Host specificity and geographical distribution of acanthocephalan parasites infecting freshwater Gammarus. Infection, Genetics and Evolution, 11, 1083–1090. https://doi.org/10.1016/j.meegid.2011.03.024