Abstract
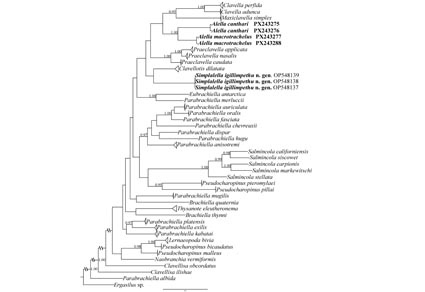
The erection of a new genus to accommodate Alella igillimpethu Erasmus, Hadfield, Wepener & Smit, 2023 as Simplalella igillimpethu gen. nov. et comb. nov. is proposed, based on molecular and morphological analyses. The new genus is closely related in the phylogenetic tree to Clavellotis dilatata (Krøyer, 1863) but according to genetic divergence, it is more closely related to Parabrachiella merlucci (Bassett-Smith, 1896), Praeclavella nasalis Castro Romero, Montes & Martorelli, 2022, and Praeclavella caudata (Castro Romero & Baeza- Kuroki, 1985) (19%). A. macrotrachelus (Brian, 1906) and A. canthari (Heller, 1865) are closely related, with 16% genetic divergence between them. Despite their confirmed validity, the cryptic species within the genus show no discernible morphological differences. A revision of the other species currently included in Alella Leigh-Sharpe, 1925, based on molecular data, is recommended to clarify their true taxonomic position. The molecular analysis also reveals a novel placement of Clavellisa Wilson C.B., 1915, which appears basal to all other analyzed Lernaeopodidae, whereas in previous phylogenies of Lernaeopodidae it was placed at the base of the Clavella Oken, 1815 branch. This new position could be explained by the presence of a short uropod in Clavellisa, along with vestigial thoracic legs in some species—features that suggest a primitive condition.
References
- Barnard, K.H. (1955) South African parasitic Copepoda. Annals of the South African Museum, 41 (5), 223–312.
- Bassett-Smith, P.W. (1896) Notes on the parasitic Copepoda of fish obtained at Plymouth, with description of new species. Annals Magazine Natural History, Series 6, 18 (103), 8–16. https://doi.org/10.1080/00222939608680404
- Ben Hassine, K., Essafi, K. & Raibaut, A. (1978) Les lernaeopodidés, copépodes parasites de Sparidae de Tunisie. Archives de l’Institut Pasteur de Tunis, 55 (4), 431–454.
- Beneden, P.J. van (1891) Deux Lernaeopodiens nouveaux recueillis l’un aux Açores, l’autre sur les côtes du Sénégal. [Two new Lernaeopodians collected, one in the Azores, the other on the coast of Senegal.] Bulletin de l’Académie Royale de Belgique, Series 3, 22, 23–35, pls. 1–2.
- Benkirane, O., Coste, F. & Raibaut, A. (1999) On the morphological variability of the attachment organ of Lernaeopodidae (Copepoda: Siphonostomatoida). Folia Parasitologica, 46 (1), 67–75.
- Blainville, N.H.D. (1822) Mémoire sur les Lernées (Lernaea, Lin.). Journal de Physique, de Chimie, d’Histoire Naturelle et des Arts, Paris, 95, 372–380 + 437–447, pl. 1.
- Boxshall, G.A. & Halsey, S.H. (2004) An Introduction to Copepod Diversity. Ray Society, London, 966 pp. https://doi.org/10.5962/bhl.title.49391
- Brian, A. (1906) Copepodi Parassiti dei Pesci d’Italia. Istituto Sordomuti, Genova, 187 pp., 21 pls. https://doi.org/10.5962/bhl.title.58642
- Castro Romero, R. & Baeza Kuroki, H. (1984) Clavellotis, new genus (Copepoda: Lernaeopodidae), and redescription of Clavellotis dilatata (Krøyer, 1863). Journal of Crustacean Biology, 4 (4), 688–694. https://doi.org/10.2307/1548082
- Castro Romero, R., & Baeza Kuroki, H. (1985) Clavella simplex sp. nov. (Copepoda, Lernaeopodidae), a parasite of Isacia conceptionis (Pisces, Teleostei) in Northern Chile. Crustaceana, 49 (2), 173–176. https://doi.org/10.1163/156854085X00413
- Castro Romero, R. & Baeza Kuroki, H. (1987) Four new species of Neobrachiella (Copepoda: Lernaeopodidae), parasitic on Sciaena genus (Teleostei: Sciaenidae) in the South Pacific. Estudios Oceanológicos, Universidad de Antofagasta, Chile, 6, 1–24, figs. 1–52, tab. 1.
- Castro Romero, R & Baeza Kuroki, H. (1989) Neobrachiella anisotremi (Copepoda, Lernaeopodidae), a new species parasites on an inshore fish, Anisotremus scapularis, off the Chilean coast. Procedings of Biological Society of Washington, 102 (1), 106–108. https://doi.org/10.1016/j.actatropica.2017.05-025
- Castro Romero, R., Montes, M.M. & Martorelli, S. (2022) Maxiclavella and Praeclavella (Siphonostomatoida: Lernaeopodidae) new genera confirmed by molecular and morphological evidence. Anais da Academia Brasileira de Ciências, 94 (4), 1–20. https://doi.org/10.1590/0001-3765202220200992
- Castro Romero, R., Montes, M., Martorelli, S.R., Sepúlveda, D., Tapia, S. & Martinez-Aquino, A. (2016) Integrative taxonomy of Peniculus, Metapeniculus and Trifur (Siphonostomatoida: Pennellidae) copepod parasites of marine fishes from Chile: species delimitation analysis using DNA barcoding evidence. Systematics and Biodiversity, 14 (5), 1–18. https://doi.org/10.1080/14772000.2016.1158213
- Cuvier, G. (1830) Le regne animnal distribute d´aprés son organisations, pour servir de base a L´histoire natrurella des animaux et d´introductiona l´a antomie comparée. Deterville, Paris, XVI + 504 pp. https://doi.org/10.5962/bhl.title.1964
- Dana, J.D. (1852) Conspectus Crustaceorum que in orbis terrarum circumnavigatione Carolo Wilkes e Classae republicae foederate duco. Paris II. Proceedings American. Academy of Arts and Science, 2, 9–61.
- Dippenaar, S.M. (2016) Does Alella Leigh-Sharpe, 1925 (Siphonostomatoida: Lernaeopodidae) really consist of seven species? Systematic Parasitology, 93 (1), 47–56. https://doi.org/10.1007/s11230-015-9601-0
- Dippenaar, S.M., Mathibela, R.B. & Bloomer, P. (2010) Cytochrome oxidase I sequences reveal possible cryptic diversity in the cosmopolitan symbiotic copepod Nesippus orientalis Heller, 1868 (Pandaridae: Siphonostomatoida) on elasmobranch hosts from the Kwa Zulu-Natal coast of South Africa. Experimental Parasitology, 125 (1), 42–50. https://doi.org/10.1016/j.exppara.2009.08.017
- Dippenaar, S.M., Narváez, K., Osaer, F. & Mangena, T. (2021) Symbiotic Siphonostomatoida (Copepoda) of the hammerhead shark species Sphyrna zygaena (Carcharhiniformes: Sphyrnidae) and stingray Dasyatis pastinaca (Myliobatiformes: Dasyatidae) off the Canary Islands, with a re-description of Pseudocharopinus pillaii Kabata, 1979. Parasitology Research, 120 (11), 3739–3747. https://doi.org/10.1007/s00436-021-07332-3
- Erasmus, A., Hadfield, K.A., Wepener, V. & Smit, N.J. (2023) Morphological and molecular characterization of Alella igillimpethu n. sp. (Copepoda: Siphonostomatoida: Lernaeopodidae) parasitizing the southern African endemic intertidal klipfish, Clinus superciliosus. Systematic Parasitology, 100 (1), 69–83. https://doi.org/10.1007/s11230-022-10071-3
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3 (5), 294–299. https://doi.org/10.1006/jmbi.1994.1499
- Forrester, G.E., McCaffrey, M.T., Terpis, K.X. & Lane, C.E. (2021) Using DNA barcoding to identify host-parasite interactions between cryptic species of goby (Coryphopterus: Gobiidae, Perciformes) and parasitic copepods (Pharodes tortugensis: Chondracanthidae, Cyclopoida). Zootaxa, 5048 (1), 99–117. https://doi.org/10.11646/zootaxa.5048.1.5
- Heller, C. (1865) Crustaceen. Reise der Österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859, Zoologie, 2, pp. 1–280, pls. 1–25.
- Hesse, M. (1863) Recherches sur quelques crustacés rares ou nouveaux des Cotes de France. Annals Science Natural, Zoologia, Series 5, 5, 265–270, pl. 9.
- Ho, J-S. (1983) Copepodes parasites of Japanese surfperches: their inference on the phylogeny and Biogeography of Embiotocidae in the far east. Annual report of the Sado Marine Biological Station Niigata University, 13, 31–62.
- Jörger, K.M. & Schrödl, M. (2013) How to describe a cryptic species? Practical challenges of molecular taxonomy. Frontiers in Zoology, 10, 59. https://doi.org/10.1186/1742-9994-10–59
- Kabata, Z. (1964) The morphology and taxonomy of Clavellodes pagelli (Krøyer, 1863) (Copepoda Lernaeopodidae). Crustaceana, 7, 103–112. https://doi.org/10.1163/156854064X00344
- Kabata, Z. (1968) Copepoda parasitic on Australian fises VIII. Families Lernaeopodidae and Naobranchiidae. Journal of Natural History, 2, 505–523. https://doi.org/10.1080/00222936800771001
- Kabata, Z. (1979) Parasitic Copepoda of British fishes. The Ray Society, London, 468 pp.
- Kabata, Z. (1986) Redescriptions of and comments on four little-known Lernaeopodidae (Crustacea: Copepoda). Canadian Journal of Zoology, 64, 1852–1859. https://doi.org/10.1139/z86-276
- Kabata, Z. (1990) Revision of the genus Clavellopsis Wilson, 1915 (Copepoda: Lernaeopodidae). Canadian Journal of Zoology, 68, 2564–2566. https://doi.org/10.1139/z90-357
- Kabata, Z, Raibaut, A. & Ben Hassine, O.K. (1971) Eubrachiella mugilis n.sp. un copepode parasite des muges de Tunisie. Bulletin de l´institute National Scientifique et Technoques du Occeanographie et de Peche, Salammbo, Nouvelle Serie, 2, 87–93.
- Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1160. https://doi.org/10.1093/bib/bbx108
- Kawatow, K., Muroga, K., Izawa, K. & Kasahara, S. (1980) Life cycle of Alella macrotrachelus (Copepoda) parasitic on cultured black sea-bream. Journal of the Faculty of Applied Biological Science, Hiroshima University, 19, 199–214.
- Krøyer, H. (1837) Om snyltekrebsene isaer med. Hensyn til dep danske fauna. Naturhistorisk Tidsskrift, 1837, 172–208 + 252–300 + 476–505 + 605–628.
- Krøyer, H. (1863-1864) Bidrag til kundskab om snyltekrensene. Naturhistorisk Tidsskrift, ser. 3,2, 75–329.
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
- Lanfear, R., Calcott, B., Kainer, D., Mayer, C. & Stamatakis, A. (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evolutionary Biology, 14, 1–82. https://doi.org/10.1186/1471-2148-14-82
- Leigh-Sharpe, W.H. (1925) A revision of the British species of Clavella (Crustacea: Copepoda) with a diagnosis of new species: C. devastatix and C. invicta. Parasitology, 17, 194–200. https://doi.org/10.1017/S0031182000004583
- Leigh-Sharpe, W.H. (1930) Chondracanthus neali n. sp. a parasitic copepod of Malacocephalus laevis. Parasitology, 22, 468–470. https://doi.org/10.1017/S0031182000011318
- Luque, J.L. & Farfán, C.A. (1991) Copépodos de la familia Lernaeopodidae (Siphonostomatoida) parásitos de algunos peces de la familia Sciaenidae (Teleotei) en el mar Peruano. Parasitología al Dia, 14, 63–66.
- Markewitsch, A.P. (1936) Nectobrachia indivisa (Copepoda, Parasit.) osoblivosti ii budovi ta sistematichne polozhenye [Nectobrachia indivisa (Copepoda, paras.), its structural features and systematic position]. Naukov і Zapiski Kyyivсьkogo Derzhavnoho Universitet, 5, 15–220.
- Montes, M.M., Castro Romero, R. & Martorelli, S.R. (2017) Morphological identification and DNA barcoding of a new species of Parabrachiella (Siphonostomatoida: Lernaeopodidae) with aspects of their intraspecific variation. Acta Tropica, 173, 34–44. https://doi.org/10.1016/j.actatropica.2017.05.025
- Montes, M.M., Castro Romero, R., Öktener, A., Balcazar, D., Reig Cardarella, G.F. & Martorelli, S.R. (2022) Molecular evidence supports the validity of three Parabrachiella species infecting mugilids. Acta Tropica, 225, 106–211. https://doi.org/10.1016/j.actatropica.2021.106211
- Montes, M.M., Gómez, S., Castro, R.R., Bovcon, N., Vettorazzi, R.I., Serrano, C.F., Cardarella, G.F.R., Ferrari, W., Cuevas, J.M. & Martorelli, S.R. (2023) How many species of genus Lernaeopoda Blainville, 1822 (Siphonostomatoida: Lernaeopodidae) are there in the southwestern Atlantic. Zootaxa, 5346 (4), 469–488. https://doi.org/10.11646/zootaxa.5346.4.6
- Muñoz G. & Castro Romero, R. (2022) Two New Parasitic Copepod Species, Clavella (Lernaeopodidae) and Haemobaphes (Pennellidae), on the Nototheniid Fish Patagonotothen cornucola (Richardson, 1844) from the Strait of Magellan, Southern Chile. Acta Parasitologica, 67 (2), 740–751. https://doi.org/10.1007/s11686-022-00517-5
- Murray, H.M., Hanlon, J.M., Marshall, K. & Morris, C.A. (2020) Redescription of the bulla, antennae, and mouth parts of female Clavella sp. (Copepoda: Siphonostomatoida: Lernaeopodidae) infesting wild Gadus morhua using scanning electron microscopy (SEM). Journal of Parasitology Research, 22, 8891448. https://doi.org/10.1155/2020/8891448
- Nei, M. & Kumar, S. (2000) Molecular evolution and phylogenetics. Oxford University Press, New York, New York, 333 pp. https://doi.org/10.1093/oso/9780195135848.001.0001
- Nordmann, A. von (1832) Mikrographisch Bitrage zur Narurgeschte der Wirbellosen Thiere. Heft 2. G. Reimer, Berlin, XVIII + 150 pp.
- Nuñes-Ruivo, L. (1966) Le Genre Alella Leigh Sharpe, 1925 (Copepoda, Fam. Lernaeopodidae). Proceedings of the 1st International Congress of Parasitology, 2, 1081–1082. https://doi.org/10.1016/b978-0-08-011427-9.50376-0
- Oken, L. (1815-1816) Lehrbuch der Naturgeschichte. Dritter Theil: Zoologie. C.H. Reclam, Leipzig and A. Schmid, Jena, xxviii + 842 + xviii pp., 40 pls., L (copepods 180–184, 357–359, 4 pls.) https://doi.org/10.5962/bhl.title.166403
- Raibaut, A & Essafi, K. (1979) Description de deus especies nouvelels de copepodes parasites des Selachiens de Tunisie. Bulletin du Museum Nationel d´histoire naturell, Paris, 4e Series, 1 Section A, 2, 435–443. https://doi.org/10.5962/p.283202
- Rangnekar, M.P. (1956) Parasitic Copepods from the marine fishes of Bombay. Jounal of the University of Bombay, Series B, 24 (5), 42–65.
- Rangnekar, M.P. (1957) Copepod parasites of the families Argulidae. Caligidae Dichelesthiidae and Lernaeopodidae. Journal of the University of Bombay, Series B, 26 (3), 8–20 + 1–6.
- Rangnekar, M.P. (1962) Copepods parasitic on fishes of Bombay. I. Lernaeopodidae. Journal of University of Bombay, 29, 193–205.
- Piasecki, W., Kempter, J., Sarabia, A. & Baptista, A. (2016) The identity of two morphotypes of Alella macrotrachelus (Brian, 1906) (Copepoda: Siphonostomatoida: Lernaeopodidae) from white Seabream, Diplodus sargus (Actinopterygii: Sparidae). Annals of Parasitology, 62. Available from; https://bibliotekanauki.pl/articles/6306 (accessed 7 September 2025)
- Pillai, N.K. (1962) Copepods parasitic on South Indian fishes families Lernaepodidae and Naobranchiidae. Journal of Marine and Biological Association, India, 4, 58–94.
- Quidor, A. (1906) Sur les copepodes recuillis par la misión Charcot dans le mers Antartiques. Bulletin du Muséum National d’Histoire Naturelle, Paris, 12, 27–33.
- Raupach, M.J., Barco, A., Steinke, D., Beermann, J., Laakmann, S., Mohrbeck, I., Neumann, H., Kihara, T.C., Pointner, K., Radulovici, A., Segelken-Voigt, A., Wesse, C. & Knebelsberger, T. (2015) The application of DNA barcodes for the identification of marine crustaceans from the North Sea and adjacent regions. PLoS ONE, 10 (9), e0139421. https://doi.org/10.1371/journal.pone.0139421
- Richiardi, S. (1880) Contribuzioni alla Fauna d’Italia. A. Catalogo sistematico dei Crostacei che vivono sul corpo degli animali. Catalogo generale della sezione Italiana all Esposizione internzionale della Pesca in Berlino, 1880, 147–152.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61 (3), 539–542. https://doi.org/10.1093/sysbio/sys029
- Ruiz, C.F., Driggers, W.B. & Bullard, S.A. (2019) A New Species of Neoalbionella (Copepoda: Siphonostomatoida: Lernaeopodidae) From Skin of the Gulper Shark, Centrophorus granulosus (Squaliformes: Centrophoridae) In the Northern Gulf of Mexico. Journal of Parasitology, 105 (2), 203–221. https://doi.org/10.1645/18-113
- Schwarz, G. (1978) Estimating the dimension of a model. Annals of Statistics, 6 (2), 461–464. https://doi.org/10.1214/aos/1176344136
- Shedko, M.M. & Shedko, S.V. (2002) Parasitic copepoda of the genus Salmincola (Lernaeopodidae) from Far Eastern chars Salvelinus (Salmonidae) with a description of a new species S. markewitschi sp.n. Zoologicheskii Zhurnal, 81, 141–153. [in Russian with English abstract]
- Shedko, S.V., Shedko, M.B., Miroshnichenko, I.L. & Nemkova, G.A. (2023) DNA Identification of Parasitic Copepods Salmincola (Copepoda, Siphonostomatoida, Lernaeopodidae): Variability and Rate of Evolution of the Mitochondrial Cytochrome c Oxidase Subunit I Gene. Russian Journal of Genetics, 59, 1022–1031. https://doi.org/10.1134/S1022795423100113
- Shiino, S.M. (1956) Copepods parasitic on Japanese fishes. 12. Lernaeopodidae. Reports Faculty of Fisheries Prefectural University Mie, 2, 269–311.
- Smith, S.I. (1874) The Crustacea of the fresh waters of the United States. Reports of the United States Fisheries Commission, 2, 637–665, pls. 1–3.
- Strøm, H. (1762) Physisk.org. economisk Beskriselve over Fogderiet sond mor, beligende I Bergens Sthf I Norge [Physiological and economic description of the Soundmorwards, located in the Diocese of Bergen in Norway]. Foste Part. Trykt hos J. Lindren, Sorøe and Rothe, Kiøbenhavn, 570 pp., tabs. 1–4., map 1.
- Van Niekerk, J.P. & Olivier, P.A.S. (1995) Alella gibbosa, a new species of Lernaeopodidae (Copepoda) from lakes St. Lucia, South Africa. South Africa Journal of Science, 91, 41–44. https://doi.org/10.17159/sajs.1995/4508
- Wilson, C.B. (1915) North American parasitic copepods belonging to the Lernaeopodidae, with a revision of the entire family. Proceedings of the United States National Museum, 47 (2063), 565–729, pls. 25–56, figs. 1–15. https://doi.org/10.5479/si.00963801.47-2063.565
- Wilson, C.B. (1935) Parasitic copepods from the Pacific Coast. The Annals and Magazine of Natural History, 16 (5), 776–797. https://doi.org/10.2307/2420107
- Yamaguti, S. (1939) Parasitic copepods from fishes of Japan. Part 6. Lernaepodidae. Volumen Jubliare pro Professore Sadao Yoshida, 2, 229–578.