Abstract
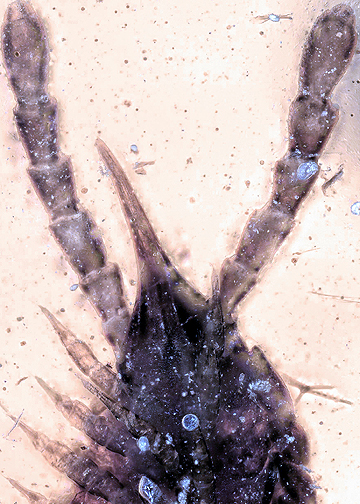
The group Neuropteriformia (beetles, lacewings, etc.) is today very species-rich, but also has a good fossil record in the Mesozoic. Amber provides not only adults, but also fossil larvae; some of these fossil neuropteriformian larvae have very unusual morphologies not seen in the modern fauna. We here report an unusual new fossil neuropteriformian larva. The mouthparts form a beak. Fossil larvae with similar mouthparts are known, and it seems that this new larva is a representative of the species ?Partisaniferus edjarzembowskii. The new larva, unlike the already known ones, has a large and inflated trunk. Based on comparison with extant larvae, such an inflated trunk should be considered physogastric. The new larva is only the second case of physogastry in fossil holometabolan larvae. Also early larvae of this species are known. The strong difference between the different larval stages give reason to interpret the ontogeny hypermetamorphic. Also this phenomenon is in fact very rare in the fossil record; most earlier candidates remain assumptions without further substantiation. Physogastry in larvae is often coupled to a mode of live in confined spaces, for a fossil preserved in amber this may mean living inside wood. Feeding mode might have been predatory, but could also have been feeding on fungi.
References
- Ardila-Camacho, A., Machado, R.J.P. & Contreras-Ramos, A. (2021) A review of the biology of Symphrasinae (Neuroptera: Rhachiberothidae), with the description of the egg and primary larva of Plega Navás, 1928. Zoologischer Anzeiger, 294, 165–185. https://doi.org/10.1016/j.jcz.2021.08.007
- Arndt, E. (1993) Phylogenetische Untersuchungen larvalmorpho-logischer Merkmale der Carabidae (Insecta: Coleoptera). Stuttgarter Beiträge zur Naturkunde, Serie A, 488, 1–56.
- Aspöck, U. & Aspöck, H. (1999) Kamelhälse, Schlammfliegen, Ameisenlöwen. Wer sind sie? (Insecta: Neuropterida: Raphidioptera, Megaloptera, Neuroptera). Stapfia, 60, 1–34.
- Aspöck, U. & Aspöck, H. (2007) Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia, 20, 451–516.
- Aspöck, U. & Aspöck, H. (2008) Phylogenetic relevance of the genital sclerites of Neuropterida (Insecta: Holometabola). Systematic Entomology, 33, 97–127. https://doi.org/10.1111/j.1365-3113.2007.00396.x
- Badano, D., Engel, M.S., Basso, A., Wang, B. & Cerretti, P. (2018) Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Communications, 9, 3257. https://doi.org/10.1038/s41467-018-05484-y
- Badano, D., Di Giulio, A., Aspöck, H., Aspöck, U. & Cerretti, P. (2021b) Burrowing specializations in a lacewing larva (Neuroptera: Dilaridae). Zoologischer Anzeiger, 293, 247–256. https://doi.org/10.1016/j.jcz.2021.06.014
- Badano, D., Fratini, M., Maugeri, L., Palermo, F., Pieroni, N., Cedola, A., Haug, J.T., Weiterschan, T., Velten, J., Mei, M., Di Giulio, A. & Cerretti, P. (2021a) X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Systematic Entomology, 46, 672–684. https://doi.org/10.1111/syen.12482
- Bahmer, H. & Lückmann, J. (2021) Zur Biologie und Ökologie von Stenoria analis Schaum, 1859 (Coleoptera: Meloidae) Ergebnisse einer fünfjährigen Untersuchung des Seidenbienen-Ölkäfers im Botanischen Garten Gießen. Oberhessische Naturwissenschaftliche Zeitschrift, 69, 7–57.
- Baranov, V.A., Wang, Y., Gašparič, R., Wedmann, S. & Haug, J.T. (2020) Eco-morphological diversity of larvae of soldier flies and their closest relatives in deep time. PeerJ, 8, e10356. https://doi.org/10.7717/peerj.10356
- Batelka, J., Engel, M.S. & Prokop, J. (2021) The complete life cycle of a Cretaceous beetle parasitoid. Current Biology, 31, R118–R119. https://doi.org/10.1016/j.cub.2020.12.007
- Beerendra, P.N., Ganguli, J. & Ganguli, R.N. (2022) Feeding efficiency of the larval stages of green lace wing, Chrysoperla zastrowi sillemi (Esben-Petersen) (Chrysopidae: Neuroptera) fed on eggs of diamond back moth of cabbage, Plutella xylostella (L.). The Pharma Innovation Journal, SP-11 (8) 2111–2113.
- Beutel, R.G., Friedrich, F. & Aspöck, U. (2010) The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zoological Journal of the Linnean Society, 158, 533–562. https://doi.org/10.1111/j.1096-3642.2009.00560.x
- Body, M., Burlat, V. & Giron, D. (2015) Hypermetamorphosis in a leaf-miner allows insects to cope with a confined nutritional space. Arthropod-Plant Interactions, 9, 75–84. https://doi.org/10.1007/s11829-014-9349-5
- Bologna, M.A. & Di Giulio, A. (2011) Biological and morphological adaptations in the pre-imaginal phases of the beetle family Meloidae. Atti Accademia Nazionale Italiana di Entomologia, 59, 141–152.
- Brito, R., Goncalves, G.L., Vargas, H.A. & Moreira, G.R. (2013) A new Brazilian Passiflora leafminer: Spinivalva gaucha, gen. n., sp. n. (Lepidoptera, Gracillariidae, Gracillariinae), the first gracillariid without a sap-feeding instar. ZooKeys, 291, 1–26. https://doi.org/10.3897/zookeys.291.4910
- Brues, C.T. (1905) Notes on the life history of the Stylopidae. The Biological Bulletin, 8, 290–295. https://doi.org/10.2307/1535804
- Burakowski, B. (1989) Hypermetamorphosis of Rhacopus attenuatus (Maeklin) (Coleoptera, Eucnemidae). Annales Zoologici, 42 (5), 165–180.
- Capelle, K.J. (1966) Observations on the life history of Ogcodes rufoabdominalis in Northern Utah (Diptera: Acroceridae). Journal of the Kansas Entomological Society, 39, 641–649.
- Chang, Y., Fang, H., Shih, C., Ren, D. & Wang, Y. (2018); Reevaluation of the subfamily Cretanallachiinae Makarkin, 2017 (Insecta: Neuroptera) from Upper Cretaceous Myanmar amber. Cretaceous Research, 84, 533–539. https://doi.org/10.1016/j.cretres.2017.10.028
- Chaudhuri, P.K. & Mazumdar, A. (2000) On the biology of Halictophagus australensis Perkins, 1905 from India (Strepsiptera, Halictophagidae). Deutsche Entomologische Zeitschrift, 47 (2), 203–215. https://doi.org/10.1002/dez.200000022
- Cruickshank, R.D. & Ko, K. (2003) Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences, 21, 441–455. https://doi.org/10.1016/S1367-9120(02)00044-5
- Darling, D.C. & Miller, T.D. (1991) Life history and larval morphology of Chrysolampus (Hymenoptera: Chalcidoidea: Chrysolampinae) in western North America. Canadian Journal of Zoology, 69, 2168–2177. https://doi.org/10.1139/z91-303
- Davis, D.R. & De Prins, J. (2011) Systematics and biology of the new genus Macrosaccus with descriptions of two new species (Lepidoptera, Gracillariidae). ZooKeys, 98, 29–82. https://doi.org/10.3897/zookeys.98.925
- Davis, D.R., Farfán, J., Cerdeña, J., Huanca-Mamani, W., Vargas, H.A., Vargas-Ortiz, M., Gonçalves, G.L. & Moreira, G.R.P. (2020) Adenogasteria leguminivora Davis & Vargas gen. et sp. nov.(Lepidoptera: Gracillariidae): a new seed‐feeding micromoth associated with Fabaceae in Peru and Chile. Austral Entomology, 59 (1), 37–51. https://doi.org/10.1111/aen.12439
- Davis, D.R. & Wagner, D. (2011) Biology and systematics of the New World Phyllocnistis Zeller leafminers of the avocado genus Persea (Lepidoptera, Gracillariidae). ZooKeys, 97, 39–73. https://doi.org/10.3897/zookeys.97.753
- Di Giulio, A., Aberlenc, H.P., Taglianti, A.V. & Bologna, M.A. (2003) Definition and description of larval types of Cyaneolytta (Coleoptera Meloidae) and new records of their phoretic association with Carabidae (Coleoptera). Tropical Zoology, 16 (2), 165–187. https://doi.org/10.1080/03946975.2003.10531193
- Engel, M.S., Barden, P., Riccio, M.L. & Grimaldi, D.A. (2016) Morphologically specialized termite castes and advanced sociality in the Early Cretaceous. Current Biology, 26, 522–530. https://doi.org/10.1016/j.cub.2015.12.061
- Fischer, T.C. (2021) In search for the unlikely: Leaf-mining caterpillars (Gracillariidae, Lepidoptera) from Upper Cretaceous and Eocene ambers. Zitteliana, 95, 135–145. https://doi.org/10.3897/zitteliana.95.63317
- Fitzgerald, T.D. (1973) Coexistence of three species of bark-mining Marmara (Lepidoptera: Gracillariidae) on green ash and descriptions of new species. Annals of the Entomological Society of America, 66, 457–464. https://doi.org/10.1093/aesa/66.2.457
- Fitzgerald, T.D. & Simeone, J.B. (1971a) Description of the immature stages of the sap feeder Marmara fraxinicola (Lepidoptera: Gracillariidae). Annals of the Entomological Society of America, 64, 765–770. https://doi.org/10.1093/aesa/64.4.765
- Fitzgerald, T.D. & Simeone, J.B. (1971b) Serpentine miner Marmara fraxinicola (Lepidoptera: Gracillariidae) in stems of white ash. Annals of the Entomological Society of America, 64, 770–773. https://doi.org/10.1093/aesa/64.4.770
- Gauweiler, J., Haug, C., Müller, P. & Haug, J.T. (2022) Lepidopteran caterpillars in the Cretaceous: were they a good food source for early birds? Palaeodiversity, 15, 45–59. https://doi.org/10.18476/pale.v15.a3
- Gepp, J. (1984) Erforschungsstand der Neuropteren-Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). In: Gepp, J., Aspöck, H. & Hölzel, H. (Eds), Progress in World’s Neuropterology. Proceedings of the 1st International Symposium on Neuropterology (22–26 September 1980, Graz, Austria), 183–239; Graz, Austria (privately printed).
- Grebennikov, V.V. (2004) Grub-like larvae of Neuroptera (Insecta): a morphological review of the families Ithonidae and Polystoechotidae and a description of Oliarces clara. European Journal of Entomology, 101, 409–417. https://doi.org/10.14411/eje.2004.056
- Grimaldi, D.A. & Engel, M.S. (2005) Evolution of the insects. Cambridge University Press, Cambridge, UK, 772 pp.
- Guillén, M. & Heraty, J.M. (2004) Instar differences in Marmara gulosa (Lepidoptera: Gracillariidae). Annals of the Entomological Society of America, 97, 1227–1232. https://doi.org/10.1603/0013-8746(2004)097[1227:IDIMGL]2.0.CO;2
- Gumovsky, A.V. (2006) The biology and morphology of Entedon sylvestris, a larval endoparasitoid of Ceutorhynchus sisymbrii (Coleoptera: Curculionidae). Journal of Hymenoptera Research, 15, 232–250.
- Gurney, A.B. (1947) Notes on Dilaridae and Berothidae, with special reference to the immature stages of the Nearctic genera (Neuroptera). Psyche, 54, 145–169. https://doi.org/10.1155/1947/78317
- Haug, C., Haug, G.T., Zippel, A., van der Wal, S. & Haug, J.T. (2021c) The earliest record of fossil solid-wood-borer larvae—immature beetles in 99 million-year-old Myanmar amber. Palaeoentomology, 4 (4), 390–404. https://doi.org/10.11646/palaeoentomology.4.4.14
- Haug, C., Zippel, A., Hassenbach, C., Haug, G.T. & Haug, J.T. (2022a) A split-footed lacewing larva from about 100-million-year-old amber indicates a now extinct hunting strategy for neuropterans. Bulletin of Geosciences, 97, 453–464.
- https://doi.org/10.3140/bull.geosci.1861
- Haug, G.T., Haug, C., Pazinato, P.G., Braig, F., Perrichot, V., Gröhn, C., Müller, P. & Haug, J.T. (2020d) The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontologia Electronica, 23 (2), a39.
- https://doi.org/10.26879/1029
- Haug, J.T. (2019) Categories of developmental biology: Examples of ambiguities and how to deal with them. In: Fusco, G. (Ed.), Perspectives on evolutionary and developmental biology. Essays for Alessandro Minelli, Festschrift 2, Padova University Press, Padova, 93–102.
- Haug, J.T., Baranov, V., Müller, P. & Haug, C. (2021a) New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Scientific Reports, 11, 20432.
- https://doi.org/10.1038/s41598-021-99480-w
- Haug, J.T., Baranov, V., Schädel, M., Müller, P., Gröhn, P. & Haug, C. (2020e) Challenges for understanding lacewings: how to deal with the incomplete data from extant and fossil larvae of Nevrorthidae? (Neuroptera). Fragmenta Entomologica, 52, 137–167.
- https://doi.org/10.13133/2284-4880/472
- Haug, J.T., Engel, M.S., Mendes dos Santos, P., Haug, G.T., Müller, P. & Haug, C. (2022b) Declining morphological diversity in snakefly larvae during last 100 million years. Paläontologische Zeitschrift, 96, 749–780.
- https://doi.org/10.1007/s12542-022-00609-7
- Haug, J.T. & Haug, C. (2022a) Another strange holometabolan larva from Kachin amber—the enigma of the beak larva (Neuropteriformia). Palaeoentomology, 5 (3), 276–284.
- https://doi.org/10.11646/palaeoentomology.5.3.11
- Haug, J.T. & Haug, C. (2022b) 100 million-year-old straight-jawed lacewing larvae with enormously inflated trunks represent the oldest cases of extreme physogastry in insects. Scientific Reports, 12, 12760.
- https://doi.org/10.1038/s41598-022-16698-y
- Haug, J.T. & Haug, C. (2023) Oldest record of a dustywing-type larva in about 100-million-year-old amber. Palaeodiversity, 16, 141–150.
- https://doi.org/10.18476/pale.v16.a7
- Haug, J.T., Haug, G.T. & Haug, C. (2023) Reconstructing the history of lacewing diversification: shape heterochrony and core tree as tools for reconstructing evolutionary processes. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 308, 1–21.
- https://doi.org/10.1127/njgpa/2023/1126
- Haug, J.T., Haug, G.T., Zippel, A., van der Wal, S., Müller, P., Gröhn, C., Wunderlich, J., Hoffeins, C., Hoffeins, H.-W. & Haug, C. (2021b) Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects, 12, art. 860.
- https://doi.org/10.3390/insects12100860
- Haug, J.T., Müller, P. & Haug, C. (2018) The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters, 4, 31.
- https://doi.org/10.1186/s40851-018-0116-9
- Haug, J.T., Müller, P. & Haug, C. (2019a) A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zoological Letters, 5, 29.
- https://doi.org/10.1186/s40851-019-0144-0
- Haug, J.T., Müller, P. & Haug, C. (2019b) A 100-million-year old slim insectan predator with massive venom-injecting stylets - a new type of neuropteran larva from Burmese amber. Bulletin of Geosciences, 94, 431–440.
- https://doi.org/10.3140/bull.geosci.1753
- Haug, J.T., Müller, P. & Haug, C. (2020b) A 100 million-year-old snake-fly larva with an unusually large antenna. Bulletin of Geosciences, 95, 167–177.
- https://doi.org/10.3140/bull.geosci.1757
- Haug, J.T., Pazinato, P.G., Haug, G.T. & Haug, C. (2020a) Yet another unusual new type of lacewing larva preserved in 100-million-year old amber from Myanmar. Rivista Italiana di Paleontologia e Stratigrafia, 126, 821–832.
- https://doi.org/10.13130/2039-4942/14439
- Haug, J.T., Schädel, M., Baranov, V.A. & Haug, C. (2020c) An unusual 100-million-year old holometabolan larva with a piercing mouth cone. PeerJ, 8, e8661.
- https://doi.org/10.7717/peerj.8661
- Haug, J.T., van der Wal, S., Gröhn, C., Hoffeins, C., Hoffeins, H.-W. & Haug, C. (2022c) Diversity and fossil record of larvae of three groups of lacewings with unusual ecology and functional morphology: Ithonidae, Coniopterygidae and Sisyridae. Palaeontologia Electronica, 25, a14.
- https://doi.org/10.26879/1212
- Heraty, J.M. & Darling, D.C. (1984) Comparative morphology of the planidial larvae of Eucharitidae and Perilampidae (Hymenoptera: Chalcidoidea). Systematic Entomology, 9, 309–328.
- https://doi.org/10.1111/j.1365-3113.1984.tb00056.x
- Huerta, C., Martínez, I. & García-Hernández, M. (2010) Preimaginal development of Onthophagus incensus Say, 1835 (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 64, 365–371.
- https://doi.org/10.1649/0010-065X-64.4.365
- Jandausch, K., Pohl, H., Aspöck, U., Winterton. S.L. & Beutel, R.G. (2018) Morphology of the primary larva of Mantispa aphavexelte Aspöck & Aspöck, 1994 (Neuroptera: Mantispidae) and phylogenetic implications to the order of Neuroptera. Arthropod Systematics & Phylogeny, 76, 529–560.
- https://doi.org/10.3897/asp.76.e31967
- Jiang, X., Shear, W. A., Hennen, D.A., Chen, H. & Xie, Z. (2019) One hundred million years of stasis: Siphonophora hui sp. nov., the first Mesozoic sucking millipede (Diplopoda: Siphonophorida) from mid-Cretaceous Burmese amber. Cretaceous Research, 97, 34–39. https://doi.org/10.1016/j.cretres.2019.01.011
- Jordan, M.P., Langmaid, J.R. & Doorenweerd, C. (2016) Morphological difference between upperside and underside leaf-mining larvae of Phyllocnistis unipunctella (Stephens, 1834) (Lep. Gracillariidae) and its changing phenology. The Entomologist’s Record and Journal of Variation, 128, 121–127.
- Kathirithamby, J. (1989) Review of the order Strepsiptera. Systematic Entomology, 14, 41–92. https://doi.org/10.1111/j.1365-3113.1989.tb00265.x
- Kathirithamby, J. (2009) Host-parasitoid associations in Strepsiptera. Annual Review of Entomology, 54, 227–249. https://doi.org/10.1146/annurev.ento.54.110807.090525
- Komárek, S. (2003) Mimicry, aposematism and related phenomena. Mimetism in nature and the history of its study. Lincom Europa, München, 167 pp.
- Komatsu, T. (2014) Larvae of the Japanese termitophilous predator Isoscelipteron okamotonis (Neuroptera, Berothidae) use their mandibles and silk web to prey on termites. Insectes Sociaux, 61, 203–205. https://doi.org/10.1007/s00040-014-0346-6
- Labandeira, C.C., Yang, Q., Santiago-Blay, J.A., Hotton, C.L., Monteiro, A., Wang, Y.J.., Goreva, Y., Shih, C.K., Siljeström, S., Rose, T.R., Dilcher, D.L. & Ren, D. (2016) The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proceedings of the Royal Society of London B, 283, 20152893. https://doi.org/10.1098/rspb.2015.2893
- Lawrence, J.F. (2016) 17. Dascilloidea Guérin-Méneville, 1843. In: Beutel, R.G. & Leschen, R.A.B. (Eds), Handbook of Zoology. Arthropoda: Insecta. Part 38. Vol. 1. Coleoptera, Beetles. Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). 2nd edn. De Gruyter, Berlin, pp. 531–542. https://doi.org/10.1515/9783110373929-020
- Lawrence, J.F., Falin, Z.H. & Ślipiński, A. (2011) 11.8. Ripiphoridae Gemminger and Harold, 1870 (Gerstaecker, 1855). In: Leschen, R.A.B., Beutel, R.G. & Lawrence, J.F (Eds), Handbook of Zoology. Arthropoda: Insecta. Part 38. Vol. 2. Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim). De Gruyter, Berlin, pp. 538–548. https://doi.org/10.1515/9783110911213.538
- Liu, X., Shi, G., Xia, F., Lu, X., Wang, B. & Engel, M.S. (2018) Liverwort mimesis in a Cretaceous lacewing larva. Current Biology, 28, 1475–1481. https://doi.org/10.1016/j.cub.2018.03.060
- Liu, X., Zhang, W., Winterton, S.L., Breitkreuz, L.C. & Engel, M.S. (2016) Early morphological specialization for insect-spider associations in Mesozoic lacewings. Current Biology, 26, 1590–1594. https://doi.org/10.1016/j.cub.2016.04.039
- Lu, X., Wang, B. & Liu, X. (2021) New Cretaceous antlion-like lacewings promote a phylogenetic reappraisal of the extinct myrmeleontoid family Babinskaiidae. Scientific Reports, 11, 16431. https://doi.org/10.1038/s41598-021-95946-z
- Luo, C., Liu, H. & Jarzembowski, E.A. (2022) High morphological disparity of neuropteran larvae during the Cretaceous revealed by a new large species. Geological Magazine, 159, 954–962. https://doi.org/10.1017/S0016756822000176
- Maia-Silva, C., Hrncir, M., Koedam, D., Machado, R.J.P. & Imperatriz-Fonseca, V.L. (2013) Out with the garbage: the parasitic strategy of the mantisfly Plega hagenella mass-infesting colonies of the eusocial bee Melipona subnitida in northeastern Brazil. Naturwissenschaften, 100, 101–105. https://doi.org/10.1007/s00114-012-0994-1
- Malicky, H. (1984) Ein Beitrag zur Autökologie und Bionomie der aquatischen Netzflüglergattung Neurorthus (Insecta, Neuroptera, Neurorthidae). Archiv für Hydrobiologie, 101, 231–246.
- Manfredini, F., Giusti, F., Beani, L. & Dallai, R. (2007) Developmental strategy of the endoparasite Xenos vesparum (Strepsiptera, Insecta): host invasion and elusion of its defense reactions. Journal of Morphology, 268, 588–601. https://doi.org/10.1002/jmor.10540
- Mergelsberg, O. (1934) Über den Begriff der Physogastrie. Zoologischer Anzeiger, 106, 97–105.
- Minter, L.R. (1990) A comparison of the eggs and first-instar larvae of Mucroberotha vesicaria Tjeder with those of other species in the families Berothidae and Mantispidae (Insecta: Neuroptera). In: Mansell, M.W. & Aspöck, H. (Eds), Proceedings of the Third International Symposium on Neuropterology. Pretoria, South Africa (South African Department of Agricultural Development), Kruger National Park, South Africa, 3–4 February 1988, 115–129.
- Möller, A., Minter, L.R. & Olivier, P.A.S. (2006) Larval morphology of Podallea vasseana Navás and Podallea manselli Aspöck & Aspöck from South Africa (Neuroptera: Berothidae). African Entomology, 14, 1–12.
- Monserrat, V.J. (2005) Nuevos datos sobre algunas pequeñas familias de neurópteros (Insecta: Neuroptera: Nevrorthidae, Osmylidae, Sisyridae, Dilaridae). Heteropterus, Revista de Entomología, 5, 1–26.
- Moreira, G.R.P., Pollo, P., Brito, R., Gonçalves, G.L. & Vargas, H.A. (2018) Cactivalva nebularia, gen. et sp. nov. (Lepidoptera: Gracillariidae): a new Weinmannia leaf miner from southern Brazil. Austral Entomology, 57, 62–76. https://doi.org/10.1111/aen.12267
- Moritz, L., Borisova, E., Hammel, J.U., Blanke, A. & Wesener, T. (2022) A previously unknown feeding mode in millipedes and the convergence of fluid feeding across arthropods. Science Advances, 8, eabm0577. https://doi.org/10.1126/sciadv.abm0577
- Muafor, F.J., Gnetegha, A.A., Le Gall, P. & Levang, P. (2015) Exploitation, trade and farming of palm weevil grubs in Cameroon. vol. 178. Bogor Barat, Indonesia (CIFOR), 32 pp.
- Muona, J. (2010) 4.5 Eucnemidae Eschscholtz, 1829. In: Leschen, R.A.B., Beutel, R.G. & Lawrence, J.F (Eds), Handbook of Zoology. Arthropoda: Insecta. Part 38. Vol. 2. Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim). De Gruyter, Berlin, pp. 61–69. https://doi.org/10.1515/9783110911213.61
- Muona, J. & Teräväinen, M. (2020) A re-evaluation of the Eucnemidae larval characters (Coleoptera). Papéis Avulsos de Zoologia, 60 (Special-issue), e202060(s.i.).28. https://doi.org/10.11606/1807-0205/2020.60.special-issue.28
- Németh, T. & Otto, R. (2016) Notes on the bionomics of Farsus dubius (Piller & Mitterpacher, 1783) (Coleoptera: Eucnemidae: Melasinae), with observations on its hypermetamorphic development. Elateridarium, 10, 133–144.
- Ohl, M. (2011) Aboard a spider—a complex developmental strategy fossilized in amber. Naturwissenschaften, 98, 453–456.
- https://doi.org/10.1007/s00114-011-0783-2
- Otto, R.L. (2017) Eucnemid larvae of the Nearctic Region. Part VII: Description of the larvae of Nematodes penetrans (LeConte, 1852) (Coleoptera: Eucnemidae: Macraulacinae: Nematodini), with notes on its hypermetamorphic life cycle. Insecta Mundi, 0545, 1–9.
- Pérez-de la Fuente, R., Delclòs, X., Peñalver, E. & Engel, M.S. (2016) A defensive behavior and plant-insect interaction in Early Cretaceous amber—the case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Structure & Development, 45, 133–139. https://doi.org/10.1016/j.asd.2015.08.002
- Pérez-de la Fuente, R., Delclòs, X., Peñalver, E., Speranza, M., Wierzchos, J., Ascaso, C. & Engel, M.S. (2012) Early evolution and ecology of camouflage in insects. Proceedings of the National Academy of Sciences, 109, 21414–21419. https://doi.org/10.1073/pnas.1213775110
- Pinto, J.D., Bologna, M.A. & Bouseman, J.K. (1996) First-instar larvae, courtship and oviposition in Eletica: amending the definition of the Meloidae (Coleoptera: Tenebrionoidea). Systematic Entomology, 21, 63–74. https://doi.org/10.1111/j.1365-3113.1996.tb00599.x
- Pohl, H. (2002) Phylogeny of the Strepsiptera based on morphological data of the first instar larvae. Zoologica Scripta, 31, 123–134. https://doi.org/10.1046/j.0300-3256.2001.00078.x
- Prell, H. (1911) Biologische Beobachtungen an Termiten und Ameisen. Zoologischer Anzeiger, 38, 243–253.
- Prokop, J., Krzemińska, E., Krzemiński, W., Rosová, K., Pecharová, M., Nel, A. & Engel, M.S. (2019) Ecomorphological diversification of the Late Palaeozoic Palaeodictyopterida reveals different larval strategies and amphibious lifestyle in adults. Royal Society Open Science, 6 (9), 190460. https://doi.org/10.1098/rsos.190460
- Read, H.J. & Enghoff, H. (2009) The order Siphonophorida—A taxonomist’s nightmare? Lessons from a Brazilian collection. Soil Organisms, 81, 543–543.
- Redborg, K.E. (1998) Biology of the Mantispidae. Annual Review of Entomology, 43, 175–194. https://doi.org/10.1146/annurev.ento.43.1.175
- Redborg, K.E. & MacLeod, E.G. (1985) The developmental ecology of Mantispa uhleri Banks (Neuroptera: Mantispidae). Illinois Biological Monographs, 53, 1–130. https://doi.org/10.5962/bhl.title.49908
- Scholtz, C.H. & Grebennikov, V.V. (2011) 12. Scarabaeiformia Crowson, 1960. In: Beutel, R.G. & Leschen, R.A.B. (Eds), Handbook of Zoology. Arthropoda: Insecta. Part. 38. Vol. 1. Coleoptera, Beetles. Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). 2nd edn. De Gruyter, Berlin, pp. 345–366. https://doi.org/10.1515/9783110904550.345
- Scholtz, C.H., Basson, R.J. & Bologna, M.A. (2018) The phoretic association between Cyaneolytta Péringuey (Coleoptera: Meloidae) triungulins and Anthia Weber (Coleoptera: Carabidae) in Southern Africa. African Entomology, 26, 555–558. https://doi.org/10.4001/003.026.0555
- Shi, G.H., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M.C., Lei, W.Y., Li, Q.L. & Li, X.H. (2012) Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37, 155–163. https://doi.org/10.1016/j.cretres.2012.03.014
- Šípek, P. & Král, D. (2012) Immature stages of the rose chafers (Coleoptera: Scarabaeidae: Cetoniinae): a historical overview. Zootaxa, 3323 (1), 1–26. https://doi.org/10.11646/zootaxa.3323.1.1
- Snyman, L.P. & Binoy, C. (2022) Evolutionary relic or a curious coincidence? A mantisfly emerging from a mud-dauber nest. Evolutionary Ecology, 36, 421–429. https://doi.org/10.1007/s10682-022-10167-8
- Švácha, P. (1994) Bionomics, behaviour and immature stages of Pelecotoma fennica (Paykull) (Coleoptera: Rhipiphoridae). Journal of Natural History, 28, 585–618. https://doi.org/10.1080/00222939400770271
- Švácha, P. & Lawrence, J.F. (2014) 2.4. Cerambycidae Latreille, 1802. In: Leschen, R.A.B. & Beutel, R.G. & (Eds), Handbook of Zoology. Arthropoda: Insecta. Part. 38. Vol. 3. Coleoptera, Beetles. Morphology and Systematics (Phytophaga). De Gruyter, Berlin, pp. 77–177. https://doi.org/10.1515/9783110274462.77
- Tauber, C.A. & Winterton, S.L. (2014) Third instar of the myrmecophilous Italochrysa insignis (Walker) from Australia (Neuroptera: Chrysopidae: Belonopterygini). Zootaxa, 3811 (1), 95–106. https://doi.org/10.11646/zootaxa.3811.1.5
- Tillyard, R.J. (1922) The life-history of the Australian moth-lacewing, Ithone fusca, Newman (Order Neuroptera Planipennia). Bulletin of Entomological Research, 13, 205–223. https://doi.org/10.1017/S000748530002811X
- Vargas-Ortiz, M., Goncalves, G.L., Huanca-Mamani, W., Vargas, H.A. & Moreira, G.R. (2019) Description, natural history and genetic variation of Caloptilia guacanivora sp. nov. Vargas-Ortiz & Vargas (Lepidoptera: Gracillariidae) in the Atacama Desert, Chile. Austral Entomology, 58, 171–191. https://doi.org/10.1111/aen.12351
- Viswam, J.P., Lee, C.K., Morgan, H.W. & McDonald, I.R. (2019) Laboratory rearing of huhu, Prionoplus reticularis (Cerambycidae): insights into the gut microbiome. New Zealand Journal of Zoology, 46, 1–12. https://doi.org/10.1080/03014223.2018.1461117
- Vitner, J. & Král, D. (2009) Immature stages and nest construction in Synapsis yunnanus (Coleoptera: Scarabaeidae). Annales de la Société Entomologique de France, 45, 49–66. https://doi.org/10.1080/00379271.2009.10697589
- Wasmann, E. (1897) Kleine Mitteilungen. Entomologische Nachrichten, 23 (2), 25–32.
- Wedmann, S., Makarkin, V.N., Weiterschan, T. & Hörnschemeyer, T. (2013) First fossil larvae of Berothidae (Neuroptera) from Baltic amber, with notes on the biology and termitophily of the family. Zootaxa, 3716 (2), 236–258. https://doi.org/10.11646/zootaxa.3716.2.6
- White, R.A., Jr. & Franklin, R.T. (1982) External morphology of larval Thanasimus dubius (Fabricius) (Coleoptera: Cleridae). The Coleopterists Bulletin, 36, 143–152. https://doi.org/10.2307/4008038
- Yang, A.S. (2001) Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evolution & Development, 3 (2), 59–72. https://doi.org/10.1046/j.1525-142x.2001.003002059.x
- Yang, Q., Wang, Y., Labandeira, C.C., Shih, C. & Ren, D. (2014) Mesozoic lacewings from China provide phylogenetic insight into evolution of the Kalligrammatidae (Neuroptera). BMC Evolutionary Biology, 14, 126. https://doi.org/10.1186/1471-2148-14-126
- Yu, T.T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F.Y., Zhang, H.C., Wang, B. & Dilcher, D. (2019) An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences, 116, 11345–11350. https://doi.org/10.1073/pnas.1821292116
- Zhao, Z.P., Shih, C.K., Gao, T.P. & Ren, D. (2021) Termite communities and their early evolution and ecology trapped in Cretaceous amber. Cretaceous Research, 117, 104612. https://doi.org/10.1016/j.cretres.2020.104612
- Zippel, A., Haug, C., Hoffeins, C., Hoffeins, H.-W. & Haug, J.T. (2022b) Expanding the record of larvae of false flower beetles with prominent terminal ends. Rivista Italiana di Paleontologia e Stratigrafia, 128, 81–104. https://doi.org/10.54103/2039-4942/17084
- Zippel, A., Haug, C., Müller, P. & Haug, J.T. (2022a) First fossil tumbling flower beetle-type larva from 99 million-year-old amber. Paläontologische Zeitschrift, 96, 219–229. https://doi.org/10.1007/s12542-022-00608-8
- Zippel, A., Haug, C., Müller, P. & Haug, J.T. (2023) The first fossil false click beetle larva preserved in amber. Paläontologische Zeitschrift, 97, 209–215. https://doi.org/10.1007/s12542-022-00638-2