Abstract
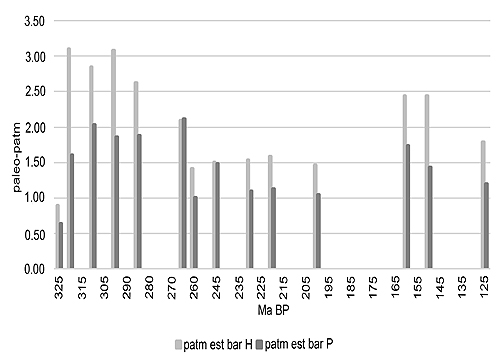
Adult Odonatoptera are among the most efficient flying predators. They have retained many physical characteristics over an immense period stretching from the Carboniferous to the present. Over this time they have greatly varied in size and mass, as shown in the fossil record and in particular by the length, shape, and structure of their wings. A fossil of Meganeurites gracilipes indicates that this large ‘griffenfly’ had a ‘hawker’ hunting behavior similar to certain extant species, with long periods of flight in which power, thermoregulation, and respiration would therefore tend to a ‘steady state’ equilibrium, allowing oxygen requirements and tracheole volumes to be projected and compared to extant ‘hawkers’. Comparing these values with standard pO2 models allows paleo-atmospheric density estimates to be derived. The results suggest that paleo-air pressure has varied from over two bars in the Late Carboniferous, Late Permian, and Middle to Late Jurassic, with lower values in the Early Triassic and Early Jurassic.
References
- Agnus, A. (1902) Description d’un Neuroptère fossile nouveau. Homoioptera gigantea. Bulletin de la Société Entomologique de France, 7, 259–261. https://doi.org/10.3406/bsef.1902.23126
- Bechly, G. (1998) A revision of the fossil dragonfly genus Urogomphus, with description of a new species. Stuttgarter Beiträge zur Naturkunde (B) Geologie und Paläontologie, 270, 1–47.
- Bechly, G., Brauckmann, C., Zessin, W. & Gröning, E. (2001) New results concerning the morphology of the most ancient dragonflies (Insecta: Odonatoptera) from the Namurian of Hagen-Vorhalle (Germany). Zeitschrift für Zoologische Systematik und Evolutionsforschung, 39, 209–226. https://doi.org/10.1046/j.1439-0469.2001.00165.x
- Bechly, G. & Makarkin, V.N. (2016) A new gigantic lacewing species (Insecta: Neuroptera) from the Lower Cretaceous of Brazil confirms the occurrence of Kalligrammatidae in the Americas. Cretaceous Research, 58, 135–140. https://doi.org/10.1016/j.cretres.2015.10.014
- Beckemeyer, R.J. (2005) Three-dimensional geometry of the wing of Megatypus schucherti (Odonatoptera: Meganeuridae). Electronic paper based on a presentation made at the FossilX3 Congress in Pretoria, South Africa, February, 2005, 18 pp.
- Beckemeyer, R.J. (2006) Hind wing fragments of Meganeuropsis (Protodonata: Meganeuridae) from the Lower Permian of Noble County, Oklahoma. Bulletin of American Odonatology, 9, 85–89.
- Belcher, C.M., Yearsley, J.M., Hadden, R.M., McElwain, J.C. & Rein, G. (2010) Baseline intrinsic flammability of Earth’s ecosystems estimated from paleoatmospheric oxygen over the past 350 million years. Proceedings of the National Academy of Sciences of the USA, 107, 22448–22453. https://doi.org/10.1073/pnas.1011974107
- Berner, R.A. (2007) Geological nitrogen cycle and atmospheric N2 over Phanerozoic time. Geology, 34, 413–415. https://doi.org/10.1130/G22470.1
- Berner, R.A. (2006) GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochimica et Cosmochimica Acta, 70, 5653–5664. https://doi.org/10.1016/j.gca.2005.11.032
- Berner, R.A. (2009) Phanerozoic atmospheric oxygen: new results using the GEOCARBSULF model. American Journal of Science, 309, 603–606. https://doi.org/10.2475/07.2009.03
- Bestwick, J., Unwin, D.M., Butler, R.J., Henderson, D.M. & Purnell, M.A. (2018) Pterosaur dietary hypotheses: a review of ideas and approaches. Biological Reviews, 93, 2021–2048. https://doi.org/10.1111/brv.12431
- Braddy, S.J., Poschmann, M. & Tetlie, O.E. (2008) Giant claw reveals the largest ever arthropod. Biology Letters, 4, 106–109. https://doi.org/10.1098/rsbl.2007.0491
- Brand, U., Davis, A.M., Shaver, K.K., Blamey, N.J.F., Heizler, M. & Lécuyer, C. (2021) Atmospheric oxygen of the Paleozoic. Earth-Science Reviews, 216, 103560. https://doi.org/10.1016/j.earscirev.2021.103560
- Bulanov, V.V. & Sennikov, A.G. (2010) New data on the morphology of Permian gliding weigeltisaurid reptiles of Eastern Europe. Paleontological Journal, 44, 682–694. https://doi.org/10.1134/S0031030110060109
- Cannell, A. (2018) The engineering of the giant dragonflies of the Permian: revised body mass, power, air supply, thermoregulation and the role of air density. Journal of Experimental Biology, 221, jeb185405. https://doi.org/10.1242/jeb.185405
- Cannell, A. (2020) Too big to fly? An engineering evaluation of the fossil biology of the giant birds of the Miocene in relation to their flight limitations, constraining the minimum air pressure at about 1.3 bar. Animal Biology, 70, 251–270. https://doi.org/10.1163/15707563-bja10001
- Clapham, M.E. & Karr, J.A. (2012) Environmental and biotic controls on the evolutionary—history of insect body size. Proceedings of the National Academy of Sciences of the United States of America, 109, 10927–10930. https://doi.org/10.1073/pnas.1204026109
- Dorrington, G.E. (2016) Heavily loaded flight and limits to the maximum size of dragonflies (Anisoptera) and griffenflies (Meganisoptera). Lethaia, 49, 261–274. https://doi.org/10.1111/let.12144
- Dudley, R. (1998) Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. Journal of Experimental Biology, 201, 1043–1050. https://doi.org/10.1242/jeb.201.8.1043
- Dudley, R. (2000) The biomechanics of insect flight: form, function, evolution. Princeton University Press, Princeton, New Jersey, xi + 476 pp. https://www.jstor.org/stable/j.ctv301g2x
- Ellington, C.P. (1985) Power and efficiency of insect flight muscle. Journal of Experimental Biology, 115, 293–304. https://doi.org/10.1242/jeb.115.1.293
- Ellington, C.P. (1999) The novel aerodynamics of insect flight: applications to micro-air vehicles. Journal of Experimental Biology, 202, 3439–3448. https://doi.org/10.1242/jeb.202.23.3439
- Falkowski, P.G., Katz, M.E., Milligan, A.J., Fennel, K., Cramer, B.S., Aubry, M.P. & Zapol, W.M. (2005) The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science, 309, 2202–2204. https://doi.org/10.1126/science.1116047
- Fleck, G., Bechly, G., Martínez-Delclòs, X., Jarzembowski, E.A., Coram, R. & Nel, A. (2003) Phylogeny and classification of the Stenophlebioptera (Odonata, Epiproctophora). Annales de la Société Entomologique de France, (N.S.), 39, 55–93. https://doi.org/10.1080/00379271.2003.10697363
- Fleck, G. & Nel, A. (2003) Revision of the Mesozoic family Aeschnidiidae (Odonata: Anisoptera). Zoologica, 153, 1–180.
- Franks, P.J., Royer, D.L., Beerling, D.J., Van de Water, P.K., Cantrill, D.J., Barbour, M.M. & Berry, J.A. (2014) New constraints on atmospheric CO2 concentration for the Phanerozoic. Geophysical Research Letters, 41, 4685–4694. https://doi.org/10.1002/2014GL060457
- Glasspool, I.J. & Scott, A.C. (2010) Phanerozoic atmospheric oxygen concentrations reconstructed from sedimentary charcoal. Nature Geoscience, 3, 627–630. https://doi.org/10.1038/ngeo692
- Goldblatt, C., Claire, M.W., Lenton, T.M., Matthews, A.J., Watson, A.J. & Zahnle, K.J. (2009) Nitrogen-enhanced greenhouse warming on early Earth. Nature Geoscience, 2, 891–896. https://doi.org/10.1038/ngeo692
- Greenlee, K.J., Henry, J.R., Kirkton, S.D., Westneat, M.W., Fezzaa, K., Lee, W.K. & Harrison J.F. (2009) Synchrotron imaging of the grasshopper tracheal system: morphological components of tracheal hypermetry and the effect of age and stage on abdominal air sac volumes and convection. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 297, 1343–1350. https://doi.org/10.1242/jeb.00767
- Harrison, J.F., Kaiser, A. & VandenBrooks, J.M. (2010) Atmospheric oxygen level and the evolution of insect body size. Proceedings of the Royal Society B, 277, 1937–1946. https://doi.org/10.1098/rspb.2010.0001
- Harrison, J.F., Waters, J.S., Cease, A.J., VandenBrooks, J.M., Callier, V., Jaco Klok, C., Shaffer, K. & Socha, J.J. (2013) How locusts breathe. Physiology, 28, 18–27. https://doi.org/10.1152/physiol.00043.2012
- Harrison, J.F., Klok, C.J. & Waters, J.S. (2014) Critical pO2 is size-independent in insects: implications for the metabolic theory of ecology. Current Opinions in Insect Science, 4, 54–59. https://doi.org/10.1016/j.cois.2014.08.012
- Harrison, J.F., Greenlee, K.J. & Verberk, W.C.E.P. (2018) Functional hypoxia in insects: definition, assessment, and consequences for physiology, ecology, and evolution. Annual Review of Entomology, 63, 303–325. https://doi.org/10.1146/annurev-ento-020117-043145
- Harrison, J.F., Wagner, J.M., Aivazian, V., Duell, M.E., Klok, C.J., Weed, M., Munoz, E., Fezzaa, K., Socha, J.J. & VandenBrooks, J.M. (2020) How to be a giant: hypermetric scaling of the leg in cockroaches and scarab beetles suggests oxygen transport to the legs limits maximal insect size. The FASEB Journal, 34, 1. https://doi.org/10.1096/fasebj.2020.34.s1.06388
- Harrison, J., Wagner, J., Aivazian, V., Duell, M., Klok, C., Weed, M., Munoz, E., Fezzaa, K., Socha, J. & VandenBrooks, J. (2021) Poor leg plumbing design saves earth from giant bugs. The FASEB Journal, 35 (Special Issue 1). https://doi.org/10.1096/fasebj.2021.35.S1.03354
- Heinrich, B. (1989) Beating the heat in obligate insect endotherms: the environmental problem and the organismal solutions. American Zoologist, 29, 1157–1168. https://doi.org/10.1093/icb/29.3.1157
- Heinrich, B. (1993) The hot-blooded insects: strategies and mechanisms of thermoregulation. Harvard University Press, Springer-Verlag Berlin, Heidelberg, v + 601 pp. https://doi.org/10.4159/harvard.9780674418516
- Garrouste, R. & Nel, A. (2014) The griffenflies (Meganisoptera, Odonatoptera) of the late Permian of Lodève (South of France): smalls and “giants”. ECOO (European Congress on Odonatology), Montpellier, France, July 2014.
- Johnson, B. & Goldblatt, C. (2018) EarthN: a new earth system nitrogen model. Geochemistry, Geophysics, Geosystems, 19, 2516–2542. https://doi.org/10.1029/2017GC007392
- Jongerius, S.R. & Lentink, D. (2010) Structural analysis of a dragonfly wing. Experimental Mechanics, 50, 1323–1334. https://doi.org/10.1007/s11340-010-9411-x
- Jouault, C., Tischlinger, H., Henrotay, M. & Nel, A. (2022) Wing coloration patterns in the Early Jurassic dragonflies as potential indicator of increasing predation pressure from insectivorous reptiles. Palaeoentomology, 5 (4), 305–318. https://doi.org/10.11646/palaeoentomology.5.4.3
- Kaiser, A., Klok, C.J., Socha, J.J., Lee, W.K., Quinlan, M.C. & Harrison, J.F. (2007) Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proceedings of the National Academy of Sciences of the United States of America, 104, 13198–13203. https://doi.org/10.1073/pnas.0611544104.
- Krause, A.J., Mills, B.J.W., Zhang, S., Planavsky, N.J., Lenton, T.M. & Poulton, S.W. (2018) Stepwise oxygenation of the Paleozoic atmosphere. Nature Communication, 9, 4081. https://doi.org/10.1038/s41467-018-06383-y
- Kuang, C., Li, Y.Z., Zhu, S. & Li, J. (2013) Influence of different low air pressure on combustion characteristics of ethanol pool fires. Procedia Engineering, 62, 226–233. https://doi.org/10.1016/j.proeng.2013.08.059
- Kukalová-Peck, J. (2009) Carboniferous protodonatoid dragonfly nymphs and the synapomorphies of Odonatoptera and Ephemeroptera (Insecta: Palaeoptera). Palaeodiversity, 2, 169–198.
- Kukalová-Peck, J. & Richardson, E.S., Jr. (1983) New Homoiopteridae (Insecta: Paleodictyoptera) with wing articulation from Upper Carboniferous strata of Mazon Creek, Illinois. Canadian Journal of Zoology, 61, 1670–1687. https://doi.org/10.1139/z83-218
- Lammer, H., Kasting, J.F., Chassefière, E., Johnson, R.E., Kulikov, Y.N. & Tian, F. (2008) Atmospheric escape and evolution of terrestrial planets and satellites. Space Science Review, 139, 399–436. https://doi.org/10.1007/s11214-008-9413-5
- Lease, H.M. & Wolf, B.O. (2010) Exoskeletal chitin scales isometrically with body size in terrestrial insects. Journal of Morphology, 271, 759–768. https://doi.org/10.1002/jmor.10835
- Mallik, A., Li, Y. & Wiedenbeck, M. (2018) Nitrogen evolution within the Earth’s atmosphere–mantle system assessed by recycling in subduction zones. Earth and Planetary Science Letters, 482, 556–566. https://doi.org/10.1016/j.epsl.2017.11.045
- May, M.L. (1982) Heat exchange and endothermy in Protodonata. Evolution, 36, 1051–1058. https://doi.org/10.1111/j.1558-5646.1982.tb05473.x
- May, M.L. (1991) Dragonfly flight: power requirements at high speed and acceleration. Journal of Experimental Biology, 158, 325–342. https://doi.org/10.1242/jeb.158.1.325
- May, M.L. (1995) Dependence of flight behavior and heat production on air temperature in the green darner dragonfly Anax junius (Odonata: Aeshnidae). Journal of Experimental Biology, 198, 2385–2392. https://doi.org/10.1242/jeb.198.11.2385
- Miller, P.L. (1994) The functions of wing clapping in the Calopterygidae (Zygoptera). Odonatologica, 23, 13–22.
- Nel, A., Béthoux, O., Bechly, G., Martínez-Delclòs, X. & Papier, F. (2001) The Permo-Triassic Odonatoptera of the ‘protodonate’ grade (Insecta: Odonatoptera). Annales de la Société Entomologique de France (N.S.), 37, 501–525.
- Nel, A., Fleck, G., Garrouste, R. & Gand, G. (2008) The Odonatoptera of the Late Permian Lodève Basin (Insecta). Journal of Iberian Geology, 34, 115–122.
- Nel, A., Fleck, G., Garrouste, R. Gand, G., Lapeyrie, J., Bybee, S.M. & Prokop, J. (2009) Revision of Permo-Carboniferous griffenflies (Insecta: Odonatoptera: Meganisoptera) based upon new species and redescription of selected poorly known taxa from Eurasia. Palaeontographica (A), 289, 89–121. https://doi.org/10.1127/pala/289/2009/89
- Nel, A. & Huang, D.Y. (2015) A new genus and species of damsel-dragonfly (Odonata: Stenophlebiidae) from the Lower Cretaceous of Inner Mongolia, China. Cretaceous Research, 56, 421–425. https://doi.org/10.1016/j.cretres.2015.06.008
- Nel, A., Martínez-Delclòs, X., Paicheler, J.-C. & Henrotay, M. (1993) Les ‘Anisozygoptera’ fossiles. Phylogénie et classification (Odonata). Martinia Numéro Hors Série, 3, 1–311.
- Nel, A., Prokop, J., Pecharová, M., Engel, M.S. & Garrouste, R. (2018) Palaeozoic giant dragonflies were hawker predators. Scientific Reports, 8 (12141). https://doi.org/10.1038/s41598-018-30629-w
- Niven, J.E. & Scharlemann, J.P.W. (2005) Do insects metabolic rates at rest and during flight scale with body mass? Biology Letters, 1, 346–349. https://doi.org/10.1098/rsbl.2005.0311
- Norberg, U.M. & Norberg, R.Å. (2012) Scaling of wingbeat frequency with body mass in bats and limits to maximum bat size. Journal of Experimental Biology, 215, 711–722. https://doi.org/10.1242/jeb.059865
- Norberg, U.M. & Rayner, J.M.V. (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society B, 316, 335–427. https://doi.org/10.1098/rstb.1987.0030
- Okajima, R. (2008) The controlling factors limiting maximum body size of insects. Lethaia, 41, 423–430. https://doi.org/10.1111/j.1502-3931.2008.00094.x
- Pennycuick, C.J. (2008) Modelling the flying bird. Elsevier Academic Press, Amsterdam, London, 496 pp.
- Petrulevičius, J.F. & Gutiérrez, P.R., (2016) New basal Odonatoptera (Insecta) from the lower Carboniferous (Serpukhovian) of Argentina. Arquivos Entomolóxicos, 16, 341–358.
- Pound, M.J., Haywood, A.M., Salzmann, U. & Riding, J.B. (2012) Global vegetation dynamics and latitudinal temperature gradients during the Mid to Late Miocene (15.97–5.33 Ma). Earth-Science Reviews, 112, 1–22. https://doi.org/10.1016/j.earscirev.2012.02.005
- Polcyn, D.M. (1988) The thermal biology of desert dragonflies. [PhD dissertation]. University of California, Riverside, California, USA.
- Pritykina, L.N. (1981) New Triassic Odonata of middle Asia. In: Vishniakova, V.N., Dlussky, G.M. & Pritykina, L.N. (Eds), Novye iskopaemye nasekomye s territorii SSSR. [New fossil insects from the territory of the U.S.S.R.]. Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, 183, 5–42.
- Prokop, J. & Nel, A. (2010) New griffenfly, Bohemiatupus elegans from the Late Carboniferous of western Bohemia in the Czech Republic (Odonatoptera: Meganisoptera: Meganeuridae). Annales de la Société Entomologique de France (N.S.), 46, 183–188. https://doi.org/10.1080/00379271.2010.10697655
- Rae, J.WB. (2018) Boron isotopes in Foraminifera: systematics, biomineralisation and CO2 reconstruction. In: Marschall, H. & Foster, G. (Eds), Boron Isotopes. Advances in Isotope Geochemistry. Springer, Cham, pp. 107–143. https://doi.org/10.1007/978-3-319-64666-4_5
- Rimmer, P.B., Shorttle, O. & Rugheimer, S. (2019) Oxidised micrometeorites as evidence for low atmospheric pressure on the early Earth. Geochemical Perspectives Letters, 9, 38–42. https://doi.org/10.7185/geochemlet.1903
- Robbins, L.L., Knorr, P.O., Wynn, J.G., Hallock, P. & Harries, P.J. (2017) Interpreting the role of pH on stable isotopes in large benthic foraminifera. ICES Journal of Marine Science, 74, 955–964. https://doi.org/10.1093/icesjms/fsw056
- Royer, D.L., Donnadieu, Y., Park, J., Kowalczyk, J. & Goddéris, Y. (2014) Error analysis of CO2 and O2 estimates from the long-term geochemical model GEOCARBSULF. American Journal of Sciences, 314, 1259–1283. https://doi.org/10.2475/09.2014.01
- Schachat, S.R., Labandeira, C.C., Saltzman, M.R., Cramer, B.D., Payne, J.L. & Boyce, C.K. (2018) Phanerozoic pO2 and the early evolution of terrestrial animals. Proceedings of the Royal Society B, 285, 20172631. https://doi.org/10.1098/rspb.2017.2631
- Schubnel, T., Legendre, F., Roques, P., Garrouste, P., Cornette, R., Perreau, M., Perreau, N., Desutter-Grandcolas, L. & Nel, A. (2021) Sound vs. light: wing-based communication in Carboniferous insects. Communications Biology, 4 (794), 1–11. https://doi.org/10.1038/s42003-021-02281-0
- Serrano, F.J., Chiappe, L.M., Palmqvist, P., Figueiredo, B., Long, J. & Sanz, J.L. (2019) The effect of long-term atmospheric changes on the macroevolution of birds. Gondwana Research, 65, 86–96. https://doi.org/10.1016/j.gr.2018.09.002
- Sharov, A.G. (1968) Filogeniya ortopteroidnykh nasekomykh. Trudy Paleontologicheskogo Instituta, Akademiya Nauk SSSR, 118, 1–216. [In Russian; translated to English in 1971: Phylogeny of the Orthopteroidea. Israel program for scientific translations, Keter Press, Jerusalem, 251 pp.]
- Sroka, P., Staniczek, A.H. & Bechly, G. (2015) Revision of the giant pterygote insect Bojophlebia prokopi Kukalová-Peck, 1985 (Hydropalaeoptera: Bojophlebiidae) from the Carboniferous of the Czech Republic, with the first cladistic analysis of fossil palaeopterous insects. Journal of Systematic Palaeontology, 13, 963–982. https://doi.org/10.1080/14772019.2014.987958
- Snelling, E.P., Seymour, R.S., Matthews, P.G. & White, C.R. (2012) Maximum metabolic rate, relative lift, wingbeat frequency and stroke amplitude during tethered flight in the adult locust Locusta migratoria. Journal of Experimental Biology, 215, 3317–3323. https://doi.org/10.1242/jeb.069799
- Suárez-Tovar, C.M. & Sarmiento, C.E. (2016) Beyond the wing planform: morphological differentiation between migratory and nonmigratory dragonfly species. Journal of Evolutionary Biology, 29, 690–703. https://doi.org/10.1111/jeb.12830
- Tillyard, R.J. (1925) The British Liassic dragonflies. British Museum (Natural History), Fossil Insects, London, 1, 1–39.
- Tracy, B.J., Tracy, C.R. & Dobkin, D.S. (1979) Desiccation in the black dragon, Hagenius brevistylus Selys. Experientia, 35, 751–752. https://doi.org/10.1007/BF01968224
- Verbeck, W.C., Bilton, D.T., Calosi, P. & Spicer, J.I. (2011) Oxygen supply in aquatic ectotherms: partial pressure and solubility together explain biodiversity and size patterns. Ecology, 92, 1565–1572. https://doi.org/10.1890/10-2369.1
- Vogt, J.R. & Dillon, M.E. (2013) Allometric scaling of tracheal morphology among bumblebee sisters (Apidae: Bombus): compensation for oxygen limitation at large body sizes? Physiological and Biochemical Zoology, 86, 576–587. https://doi.org/10.1086/672211
- Wagner, J., Duell, M. & Harrison J. (2016) Hypermetric tracheal scaling as a factor in insect gigantism. The FASEB Journal, 30, lb646. https://doi.org/10.1096/fasebj.30.1_supplement.lb646
- Wakeling, J.M. & Ellington, C.P. (1997) Dragonfly flight. III. Lift and power requirements. Journal of Experimental Biology, 200, 583–600. https://doi.org/10.1242/jeb.200.3.583
- Wang, S., Shao, L.Y., Yan, Z.M., Shi M.J. & Zhang Y.H. (2019) Characteristics of Early Cretaceous wildfires in peat-forming environment, NE China. Journal of Palaeogeography, 8, 17. https://doi.org/10.1186/s42501-019-0035-5
- Waller, J.T. & Svensson, E.I. (2017) Body size evolution in an old insect order: no evidence for Cope’s Rule in spite of fitness benefits of large size. Evolution, 71, 2178–2193. https://doi.org/10.1111/evo.13302
- Wei, Y., Pu, Z.Y., Zong, Q.G., Wan, W.X., Dubinin, E. & Fränz, M. (2014) Geomagnetic reversals, atmospheric escape and mass extinctions. Earth and Planetary Science Letters, 394, 94–98. https://doi.org/10.1016/j.epsl.2014.03.018
- Wootton, R.J. (1991) The functional morphology of the wings of Odonata. Advances in Odonatology, 5, 153–169.
- Wootton, R.J., Kukalová-Peck, J., Newman, J.S. & Muzon, J. (1998) Smart engineering in the Mid-Carboniferous: how well could Paleozoic dragonflies fly? Science, 282, 749–751. https://doi.org/10.1126/science.282.5389.749
- Wang, S., Shao, L.Y., Yan, Z.M. & Weis-Fogh, T. (1967) Respiration and tracheal ventilation in locusts and other flying insects. Journal of Experimental Biology, 47, 561–587. https://doi.org/10.1242/jeb.47.3.561
- Yoshioka, T., Wiedenbeck, M., Shcheka, S. & Keppler, H. (2018) Nitrogen solubility in the deep mantle and the origin of Earth’s primordial nitrogen budget. Earth and Planetary Science Letters, 488, 134–143. https://doi.org/10.1016/j.epsl.2018.02.021
- Zhang, H.C., Zheng, D.R., Wang, B., Fang, Y. & Jarzembowski, E.A. (2013) The largest known odonate in China: Hsiufua chaoi Zhang et Wang, gen. et sp nov from the Middle Jurassic of Inner Mongolia. Chinese Science Bulletin, 58, 1579–1584. https://doi.org/10.1007/s11434-012-5567-3
- Zhang, S., Planavsky, N.J., Krause, A.J., Bolton, E.W. & Mills, B.J.W. (2018) Model based Paleozoic atmospheric oxygen estimates: a revisit to GEOCARBSULF. American Journal of Science, 318, 557–589. https://doi.org/10.2475/05.2018.05
- Zhang, Z.J., Hong, Y.C., Lu, L.W., Fang, X.S. & Jin, Y.G. (2006) Shenzhousia qilianshanensis gen. et sp. nov. (Protodonata, Meganeuridae), a giant dragonfly from the Upper Carboniferous of China. Progress in Natural Science, 16, 328–330. https://doi.org/10.1080/10020070612331343233
- Zheng, D.R., Nel, A., Wang, H., Wang, B., Jarzembowski, E.A., Chang, S.C. & Zhang, H.C. (2017) The first Late Triassic Chinese triadophlebiomorphan (Insecta: Odonatoptera): biogeographic implications. Scientific Reports, 7, 1–7. https://doi.org/10.1038/s41598-017-01710-7
- Zhou, C.F., Gao, K.Q., Yi, H., Xue, J., Li, Q. & Fox, R.C. (2017) Earliest filter-feeding pterosaur from the Jurassic of China and ecological evolution of Pterodactyloidea. Royal Society Open Science, 4, 160672. https://doi.org/10.1098/rsos.160672