Abstract
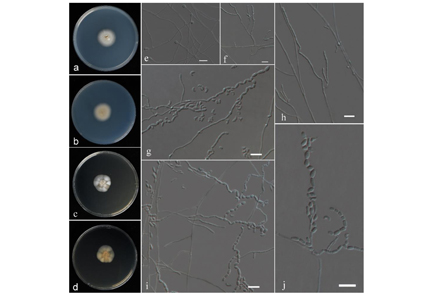
During a survey of keratinolytic fungi in China, a new species, Arthrographis multiformispora was isolated from soil samples. Morphologically, A. multiformispora differs from other species in the genus by the presence of globose or subglobose chlamydospores and cylindrical arthroconidia. Phylogenetically, our four strains were clustered together with high support values and separated from other clades. We provided a description, illustrations, and phylogenetic tree for the new species.
References
<p>Biser, S.A., Perry, H.D., Donnenfeld, E.D., Doshi, S.J. & Chaturvedi, V. (2004) <em>Arthrographis</em> <em>kalrae</em> mimicking acanthamoeba keratitis. <em>Cornea</em> 23: 314–317. https://doi.org/10.1097/00003226-200404000-00018</p>
<p>Boana, P., Arthur, I., Golledge, C. & Ellis, D. (2012) Refractory <em>Arthrographis kalrae</em> native knee joint infection. <em>Medical Mycology Case Reports</em> 1 (1): 112–114. https://doi.org/10.1016/j.mmcr.2012.10.005</p>
<p>Capella-Gutierrez, S., Silla-Martinez, J.M. & Gabaldon, T. (2009) TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. <em>Bioinformatics</em> 25: 1972–1973. https://doi.org/10.1093/bioinformatics/btp348</p>
<p>Chen, C.J., Chen, H., Zhang, Y., Thomas, H.R., Frank, M.H., He, Y.H. & Xia, R. (2020) TBtools: An integrative toolkit developed for interactive analyses of big biological data. <em>Molecular Plant</em> 13 (8): 1194–1202. https://doi.org/10.1016/j.molp.2020.06.009</p>
<p>Chin-Hong, P.V., Sutton, D.A., Roemer, M., Jacobson, M.A. & Aberg, J.A. (2001) Invasive fungal sinusitis and meningitis due to <em>Arthrographis kalrae</em> in a patient with AIDS. <em>Journal of Clinical Microbiology</em> 39: 804–807. https://doi.org/10.1128/JCM.39.2.804-807.2001</p>
<p>Giraldo, A., Gené, J., Sutton, D.A., Madrid, H., Cano, J., Crous, P.W. & Guarro, J. (2014) Phylogenetic circumscription of <em>Arthrographis</em> (<em>Eremomycetaceae</em>, <em>Dothideomycetes</em>). <em>Persoonia</em> 32: 102–114. https://doi.org/10.3767/003158514x680207</p>
<p>Hernández-Restrepo, M., Giraldo, A., van Doorn, R., Wingfield, M.J., Groenewald, J.Z., Barreto, R.W., Colmán, A.A., Mansur, P.S.C. & Crous, P.W. (2020) The genera of Fungi – G6: <em>Arthrographis</em>, <em>Kramasamuha</em>, <em>Melnikomyces</em>, <em>Thysanorea</em>, and <em>Verruconis</em>. <em>Fungal Systematics and Evolution</em> 6 (1). https://doi.org/10.3114/fuse.2020.06.01</p>
<p>Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. <em>Nature Methods</em> 14 (6): 587–589. https://doi.org/10.1038/nmeth.4285</p>
<p>Kang, H.J., Sigler, L., Lee, J., Gibas, C.F., Yun, S.H. & Lee, Y.W. (2010) <em>Xylogone</em> <em>Ganodermophthora</em> sp. nov., an ascomycetous pathogen causing yellow rot on cultivated mushroom Ganoderma lucidum in Korea. <em>Mycologia</em> 102 (5): 1167–1184. https://doi.org/10.3852/09-304</p>
<p>Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. <em>Molecular Biology and Evolution</em> 30 (4): 772–780. https://doi.org/10.1093/molbev/mst010</p>
<p>Minh, Q., Nguyen, M. & von Haeseler, A.A. (2013) Ultrafast approximation for phylogenetic bootstrap. <em>Molecular Biology and Evolution</em> 30, 1188–1195. https://doi.org/10.1093/molbev/mst024</p>
<p>Nguyen, L.T., Schmidt, H.A., von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. <em>Molecular Biology and Evolution</em> 32, 268–274. https://doi.org/10.1093/molbev/msu300</p>
<p>Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. <em>Systematic Biology</em> 61, 539–542. https://doi.org/10.1093/sysbio/sys029</p>
<p>Sigler, L. & Carmichael, J.W. (1976) Taxonomy of <em>Malbranchea</em> and some other <em>Hyphomycetes</em> with <em>arthroconidia</em>. <em>Mycotaxon</em> 4: 349–488.</p>
<p>Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. <em>Molecular Biology and Evolution</em> 30: 2725–2729. https://doi.org/10.1093/molbev/mst197</p>
<p>Tewari, R.P. & Macpherson, C.R. (1971) A new dimorphic fungus, <em>Oidiodendron kalrae</em>: morphological and biochemical characteristics. <em>Mycologia</em> 63: 602–611. https://doi.org/10.2307/3757556</p>
<p>Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several <em>Cryptococcus</em> species. <em>Journal of Bacteriology</em> 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990</p>
<p>Voigt, K. & Wöstemeyer, J. (2000) Reliable amplification of actin genes facilitates deep-level phylogeny. <em>Microbiological Research</em> 155: 179–195. https://doi.org/10.1016/s0944-5013(00)80031-2</p>
<p>White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. <em>In</em>: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) <em>PCR protocols: a guide to methods and applications</em>, Academic Press, San Diego, California, pp 315–322.</p>
<p>Yoshitsugu, S. & Masaki, H. (2010) <em>Arthrographis kalrae</em>, a rare causal agent of onychomycosis, and its occurrence in natural and commercially available soils. <em>Medical Mycology</em> 48: 2, 384–389. https://doi.org/10.3109/13693780903219014</p>
<p>Zhang, D., Gao, F.L., Jakovlić, I., Zou, H., Zhang, J., Li, W.X. & Wang, G.T. (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. <em>Molecular Ecology Resources</em> 20 (1): 348–355. https://doi.org/10.1111/1755-0998.13096</p>
<p>Zhang, Z.Y., Dong, C.B., Chen, W.H., Mou, Q.R., Lu, X.X., Han, Y.F., Huang, J.Z. & Liang, Z.Q. (2020a) The enigmatic <em>Thelebolaceae</em> (Thelebolales, Leotiomycetes): one new genus <em>Solomyces</em> and five new species. <em>Frontiers in Microbiology</em> 11: 572596. https://doi.org/10.3389/fmicb.2020.572596</p>
<p>Zhang, Z.Y., Shao, Q.Y., Li, X., Chen, W.H., Liang, J.D., Han, Y.F., Huang, J.Z. & Liang, Z.Q. (2021) Culturable fungi from Urban soils in China I: description of 10 new taxa.<em> Microbiology Spectrum</em> 9: e00867–21. https://doi.org/10.1128/Spectrum.00867-21</p>
<p>Zhang, Z.Y., Zhao, Y.X., Shen, X., Chen, W.H., Han, Y.F., Huang, J.Z., Liang, Z.Q.(2020b) Molecular phylogeny and morphology of <em>Cunninghamella guizhouensis</em> sp. nov. (Cunninghamellaceae, Mucorales), from soil in Guizhou, China. <em>Phytotaxa</em> 455 (1): 31–39. https://doi.org/10.11646/phytotaxa.455.1.4</p>
<p>Boana, P., Arthur, I., Golledge, C. & Ellis, D. (2012) Refractory <em>Arthrographis kalrae</em> native knee joint infection. <em>Medical Mycology Case Reports</em> 1 (1): 112–114. https://doi.org/10.1016/j.mmcr.2012.10.005</p>
<p>Capella-Gutierrez, S., Silla-Martinez, J.M. & Gabaldon, T. (2009) TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. <em>Bioinformatics</em> 25: 1972–1973. https://doi.org/10.1093/bioinformatics/btp348</p>
<p>Chen, C.J., Chen, H., Zhang, Y., Thomas, H.R., Frank, M.H., He, Y.H. & Xia, R. (2020) TBtools: An integrative toolkit developed for interactive analyses of big biological data. <em>Molecular Plant</em> 13 (8): 1194–1202. https://doi.org/10.1016/j.molp.2020.06.009</p>
<p>Chin-Hong, P.V., Sutton, D.A., Roemer, M., Jacobson, M.A. & Aberg, J.A. (2001) Invasive fungal sinusitis and meningitis due to <em>Arthrographis kalrae</em> in a patient with AIDS. <em>Journal of Clinical Microbiology</em> 39: 804–807. https://doi.org/10.1128/JCM.39.2.804-807.2001</p>
<p>Giraldo, A., Gené, J., Sutton, D.A., Madrid, H., Cano, J., Crous, P.W. & Guarro, J. (2014) Phylogenetic circumscription of <em>Arthrographis</em> (<em>Eremomycetaceae</em>, <em>Dothideomycetes</em>). <em>Persoonia</em> 32: 102–114. https://doi.org/10.3767/003158514x680207</p>
<p>Hernández-Restrepo, M., Giraldo, A., van Doorn, R., Wingfield, M.J., Groenewald, J.Z., Barreto, R.W., Colmán, A.A., Mansur, P.S.C. & Crous, P.W. (2020) The genera of Fungi – G6: <em>Arthrographis</em>, <em>Kramasamuha</em>, <em>Melnikomyces</em>, <em>Thysanorea</em>, and <em>Verruconis</em>. <em>Fungal Systematics and Evolution</em> 6 (1). https://doi.org/10.3114/fuse.2020.06.01</p>
<p>Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. <em>Nature Methods</em> 14 (6): 587–589. https://doi.org/10.1038/nmeth.4285</p>
<p>Kang, H.J., Sigler, L., Lee, J., Gibas, C.F., Yun, S.H. & Lee, Y.W. (2010) <em>Xylogone</em> <em>Ganodermophthora</em> sp. nov., an ascomycetous pathogen causing yellow rot on cultivated mushroom Ganoderma lucidum in Korea. <em>Mycologia</em> 102 (5): 1167–1184. https://doi.org/10.3852/09-304</p>
<p>Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. <em>Molecular Biology and Evolution</em> 30 (4): 772–780. https://doi.org/10.1093/molbev/mst010</p>
<p>Minh, Q., Nguyen, M. & von Haeseler, A.A. (2013) Ultrafast approximation for phylogenetic bootstrap. <em>Molecular Biology and Evolution</em> 30, 1188–1195. https://doi.org/10.1093/molbev/mst024</p>
<p>Nguyen, L.T., Schmidt, H.A., von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. <em>Molecular Biology and Evolution</em> 32, 268–274. https://doi.org/10.1093/molbev/msu300</p>
<p>Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. <em>Systematic Biology</em> 61, 539–542. https://doi.org/10.1093/sysbio/sys029</p>
<p>Sigler, L. & Carmichael, J.W. (1976) Taxonomy of <em>Malbranchea</em> and some other <em>Hyphomycetes</em> with <em>arthroconidia</em>. <em>Mycotaxon</em> 4: 349–488.</p>
<p>Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. <em>Molecular Biology and Evolution</em> 30: 2725–2729. https://doi.org/10.1093/molbev/mst197</p>
<p>Tewari, R.P. & Macpherson, C.R. (1971) A new dimorphic fungus, <em>Oidiodendron kalrae</em>: morphological and biochemical characteristics. <em>Mycologia</em> 63: 602–611. https://doi.org/10.2307/3757556</p>
<p>Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several <em>Cryptococcus</em> species. <em>Journal of Bacteriology</em> 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990</p>
<p>Voigt, K. & Wöstemeyer, J. (2000) Reliable amplification of actin genes facilitates deep-level phylogeny. <em>Microbiological Research</em> 155: 179–195. https://doi.org/10.1016/s0944-5013(00)80031-2</p>
<p>White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. <em>In</em>: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) <em>PCR protocols: a guide to methods and applications</em>, Academic Press, San Diego, California, pp 315–322.</p>
<p>Yoshitsugu, S. & Masaki, H. (2010) <em>Arthrographis kalrae</em>, a rare causal agent of onychomycosis, and its occurrence in natural and commercially available soils. <em>Medical Mycology</em> 48: 2, 384–389. https://doi.org/10.3109/13693780903219014</p>
<p>Zhang, D., Gao, F.L., Jakovlić, I., Zou, H., Zhang, J., Li, W.X. & Wang, G.T. (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. <em>Molecular Ecology Resources</em> 20 (1): 348–355. https://doi.org/10.1111/1755-0998.13096</p>
<p>Zhang, Z.Y., Dong, C.B., Chen, W.H., Mou, Q.R., Lu, X.X., Han, Y.F., Huang, J.Z. & Liang, Z.Q. (2020a) The enigmatic <em>Thelebolaceae</em> (Thelebolales, Leotiomycetes): one new genus <em>Solomyces</em> and five new species. <em>Frontiers in Microbiology</em> 11: 572596. https://doi.org/10.3389/fmicb.2020.572596</p>
<p>Zhang, Z.Y., Shao, Q.Y., Li, X., Chen, W.H., Liang, J.D., Han, Y.F., Huang, J.Z. & Liang, Z.Q. (2021) Culturable fungi from Urban soils in China I: description of 10 new taxa.<em> Microbiology Spectrum</em> 9: e00867–21. https://doi.org/10.1128/Spectrum.00867-21</p>
<p>Zhang, Z.Y., Zhao, Y.X., Shen, X., Chen, W.H., Han, Y.F., Huang, J.Z., Liang, Z.Q.(2020b) Molecular phylogeny and morphology of <em>Cunninghamella guizhouensis</em> sp. nov. (Cunninghamellaceae, Mucorales), from soil in Guizhou, China. <em>Phytotaxa</em> 455 (1): 31–39. https://doi.org/10.11646/phytotaxa.455.1.4</p>