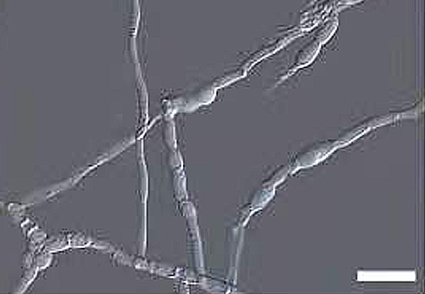
Abstract
The genus Dlhawksworthia presently includes three species. All the previously described species have been isolated from plants. Besides, none of these three species have ever been recorded in China. We conducted surveys in various regions of China to isolate and identify endolichenic fungi associated with diverse lichen species. During these surveys, we isolated both previously known and undescribed fungi associated with lichens. Among these, there was an isolate of an unknown fungus. The morphological and molecular analyses indicated that this isolate represented a new species from the genus Dlhawksworthia. As a consequence, we described this fungus as Dlhawksworthia flavoparmeliae sp. nov. This is the first report of Dlhawksworthia isolated from a lichen in China and globally.
References
<p>Arnold, A.E., Miadlikowska, J., Higgins, K.L., Sarvate, S.D., Gugger, P., Way, A., Hofstetter, V., Kauff, F. & Lutzoni, F. (2009) A phylogenetic estimation of trophic transition networks for ascomycetous Fungi: Are lichens cradles of symbiotrophic Fungal diversification? <em>Systematic Biology</em> 58: 283–297. http://doi.org/10.1093/sysbio/syp001</p>
<p>Carbone, I. & Kohn, L.M. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. <em>Mycologia</em> 91: 553–556. http://doi.org/10.2307/3761358</p>
<p>Chaiwan, N., Wanasinghe, D.N., Camporesi, E., Tibpromma, S., Boonmee, S., Lumyong, S. & Hyde, K.D. (2019) Molecular taxonomy reveals the sexual morph of <em>Nodulosphaeria digitalis</em> in Phaeosphaeriaceae from <em>Campanula trachelium</em> in Italy. <em>Phytotaxa</em> 400: 1–13. http://doi.org/10.11646/phytotaxa.400.1.1</p>
<p>Hawksworth, D.L. & Honegger, R. (1994) The lichen thallus: asymbiotic phenotype of nutritionally specialized fungi and its response to gall producers. <em>In:</em> Williams, M.A.J. (Ed.) <em>Plant galls: organisms, interactions, populations.</em> Oxford, UK: Clarendon Press, pp. 77–98.</p>
<p>Huang, S., Jeewon, R., Wanasinghe, D.N., Manawasinghe, I.S., Bulgakov, T.S., Hyde, K.D. & Kang, J. (2017) Phylogenetic taxonomy of <em>Dematiopleospora fusiformis sp. nov</em>. (Phaeosphaeriaceae) from Russia. <em>Phytotaxa</em> 316: 239–249. http://doi.org/10.11646/phytotaxa.316.3.3</p>
<p>Hyde, K.D., Hongsanan, S., Jeewon, R., Bhat, D.J., McKenzie, E.H.C., Jones, E.B.G., Phookamsak, R., Ariyawansa, H.A., Boonmee, S., Zhao, Q., Abdel-Aziz, F.A., Abdel Wahab, M.A., Banmai, S., Chomnunti, P., Cui, B.-K., Daranagama, D.A., Das, K., Dayarathne, M.C., Silva, N.I.de., Dissanayake, A.J., Doilom, M., Ekanayaka, A.H., Gibertoni, T.B., Go´es-Neto, A., Huang, S.-K., Jayasiri, S.C., Jayawardena, R.S., Konta, S., Lee, H.B., Li, W.-J., Lin, C.-G., Liu, J.-K., Lu, Y.-Z., Luo, Z.-L., Manawasinghe, I.S., Manimohan, P., Mapook, A., Niskanen, T., Norphanphoun, C., Papizadeh, M., Perera, R.H., Phukhamsakda, C., Richter, C., Santiago, A.L.C.M.de A., Drechsler-Santos, E.R., Senanayake, I.C. & Tanaka, K. (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. <em>Fungal Diversity</em> 80: 1–270. http://doi.org/10.1007/s13225-016-0373-x</p>
<p>Katoh, K., Rozewicki, J. & D. Yamada, K. (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. <em>Briefings in Bioinformatics</em> 20:1–7. https://doi.org/10.1093/bib/bbx108</p>
<p>Kumar, S., Tamura, K., Peterson, D., Peterson, N., Stecher, G. & Nei, M. (2011) MEGA5:Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. <em>Molecular Biology and</em> <em>Evolution</em> 28: 2731–2739. http://doi.org/10.1093/molbev/msr121</p>
<p>Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Dura, C., Thierer, T., Ashton, B., Meintjes, P. & Drummond, A. (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. <em>Bioinformatics</em> 28: 1647–1649. https://doi.org/10.1093/bioinformatics/bts199</p>
<p>Masumoto, H. & Degawa, Y. (2019) The effect of surface sterilization and the type of sterilizer on the genus composition of lichen-inhabiting fungi with notes on some frequently isolated genera. <em>Mycoscience</em> 60: 331–342. https://doi.org/10.1016/j.myc.2019.07.004</p>
<p>Miadlikowska, J., Arnold, A.E. & Lutzoni, F. (2004) Diversity of cryptic fungi inhabiting healthy lichen thalli in a temperate and tropical forest. <em>Ecological Society of America Annual Meet</em> 89: 349–350.</p>
<p>Miller, M.A., Pfeiffer, W.T. & Schwartz, T. (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. <em>In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010.</em> New Orleans, pp. 1–8. http://doi.org/10.1109/gce.2010.5676129</p>
<p>Mitrović, T., Stamenkovic, S., Cvetković, V., Nikolic, M., Baosic, R., Mutic, J., Andjelkovic, T. & Bojić, A. (2012) Epiphytic lichen <em>Flavoparmelia caperata</em> as a sentinel for trace metal pollution. <em>Journal of the Serbian Chemical Society</em> 77: 1301–1310. http://doi.org/10.2298/JSC111124031M</p>
<p>Nieuwenhuis, B.P.S. & James, T.Y. (2016) The frequency of sex in fungi. <em>Philosophical Transactions of the Royal Society B: Biological Sciences</em> 371: 1706. https://doi.org/10.1098/rstb.2015.0540</p>
<p>Nylander, J.A.A., Wilgenbusch, J.C., Warren, D.L. & Swofford, D.L. (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. <em>Bioinformatics</em> 24: 581–583. https://doi.org/10.1093/bioinformatics/btm388</p>
<p>Pathak, A., Mishra, R.K., Shukla, S.K., Kumar, R., Pandey, A., Pandey, M. & Dikshit, A. (2016) <em>Flavoparmelia caperata</em>, a host for <em>Beauveria</em> sp. in subalpine forest of Chakrata district, Uttarakhand, India, and natural selection in <em>B. bassiana</em>. <em>Asian Journal of Microbiology, Biotechnology and Environmental Sciences</em> 18: 981–998.Phookamsak, R., Liu, J.-K., McKenzie, E.H.C., Manamgoda, D.S., Ariyawansa, H., Thambugala, K.M., Dai, D.-Q., Camporesi, E., Chukeatirote, E., Wijayawardene, N.N., Bahkali, A.H., Mortimer, P.E., Xu, J.-C. & Hyde, K.D. (2014) Revision of <em>Phaeosphaeriaceae</em>. <em>Fungal Diversity </em>68: 159–238. https://doi.org/10.1007/s13225-014-0308-3</p>
<p>Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. <em>Bioinformatics</em> 19: 1572–1574. https://doi.org/10.1093/bioinformatics/btg180</p>
<p>Stamatakis, A., Ludwig, T. & Meier, H. (2005) RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. <em>Bioinformatics </em>21: 456–463. https://doi.org/10.1093/bioinformatics/bti191</p>
<p>Suryanarayanan, T.S. & Thirunavukkarasu, N. (2017) Endolichenic fungi: the lesser known fungal associates of lichens. <em>Mycology</em> 8: 189–196. https://doi.org/10.1080/21501203.2017.1352048</p>
<p>Tripathi, M., Joshi, Y. & Gupta, R.C. (2014) Assessment of endolichenic fungal diversity in some forests of Kumaun Himalaya. <em>Current science</em> 107: 745–748.</p>
<p>Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several <em>Cryptococcus</em> species. <em>Journal of Bacteriology</em> 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990</p>
<p>Wanasinghe, D.N. & Hyde, K.D. (2018) Nomenclatural novelties. <em>Index Fungorum</em> 357: 1.</p>
<p>Wanasinghe, D.N., Phukhamsakda, C., Hyde, K.D., Jeewon, R., Lee, B.H., Jones, E.B.G., Tibpromma, S., Tennakoon, D.S., Dissanayake, A.J., Jayasiri, S.C., Gafforov, Y., Camporesi, E., Bulgakov, T.S., Ekanayake, A.H., Perera, R.H., Samarakoon, M.C., Goonasekara, I.D., Mapook, A., Li, W.J., Senanayake, I.C., Li, J.-F., Norphanphoun, C., Doilom, M., Bahkali, A.H., Xu, J.-C., Mortimer, P.E., Tibell, L., Tibell, S. & Karunarathna, S.C. (2018) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. <em>Fungal Diversity</em> 89: 1–236. https://doi.org/10.1017/S0024282919000483</p>
<p>White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. <em>In:</em> Innis, M.A., Gelfrand, D.H., Sninsky, J.J. & White, T.J. (eds.) <em>PCR Protocols</em>. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/b978-0-12-372180-8.50052-1</p>
<p>Yoshino, K., Yamamoto, K., Masumoto, H., Degawa, Y., Yoshikawa, H., Harada, H. & Sakamoto, K. (2020) Polyol-assimilation capacities of lichen-inhabiting fungi. <em>The Lichenologist</em> 52: 49–59. https://doi.org/10.1017/S0024282919000483</p>
<p>Zhang, Y.-J., Zhang, S., Liu, X.-Z., Wen, H.-A. & Wang, M. (2010) A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. <em>Letters in Applied Microbiology </em>51: 114–118. https://doi.org/10.1111/j.1472-765X.2010.02867.x</p>
<p>Zoller, S., Scheidegger, C. & Sperisen, C. (1999) PCR primers for the amplication of mitochondrial small subunite ribosomal DNA of lichen-forming Ascomycetes. <em>The lichenologist</em> 31:511–516. https://doi.org/10.1017/S0024282999000663</p>